Article Text
Abstract
Objective Patient safety may be enhanced by using reports from front-line staff of near misses and unsafe conditions to identify latent safety events. We describe paediatric emergency department (ED) near-miss events and unsafe conditions from hospital reporting systems in a 1-year observational study from hospitals participating in the Pediatric Emergency Care Applied Research Network (PECARN).
Design This is a secondary analysis of 1 year of incident reports (IRs) from 18 EDs in 2007–2008. Using a prior taxonomy and established method, this analysis is of all reports classified as near-miss (events not reaching the patient) or unsafe condition. Classification included type, severity, contributing factors and personnel involved. In-depth review of 20% of IRs was performed.
Results 487 reports (16.8% of eligible IRs) are included. Most common were medication-related, followed by laboratory-related, radiology-related and process-related IRs. Human factors issues were related to 87% and equipment issues to 11%. Human factor issues related to non-compliance with procedures accounted for 66.4%, including 5.95% with no or incorrect ID. Handoff issues were important in 11.5%.
Conclusions Medication and process-related issues are important causes of near miss and unsafe conditions in the network. Human factors issues were highly reported and non-compliance with established procedures was very common, and calculation issues, communications (ie, handoffs) and clinical judgment were also important. This work should enable us to help improve systems within the environment of the ED to enhance patient safety in the future.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Strengths and limitations of this study
This analysis involved 18 paediatric emergency departments' incident reports (IRs), including all filed for a calendar year, representing a large sample representative of the systems at the time of study.
Each was reviewed by two investigators and categorised as near miss or unsafe conditions.
IR systems at the start of the study under-represent latent safety threats at most of the institutions.
IR systems may represent reporting culture and often in themselves are not granular enough to derive better safety outcomes because of lack of how each was followed up.
There was wide variability of reporting rates (IRs per patient visit).
Background
The reporting of near misses and unsafe conditions is important for identifying and addressing latent safety issues to prevent serious patient safety events.1 In the IOM report To Err is Human, recommendation 5.2 is for the development of voluntary reporting efforts, including the funding ‘and evaluation of pilot projects for reporting systems, both within individual healthcare organisations’ and among organisations.1 BMJ (2000) had a clinical review which described the role of non-medical high-risk industries’ use of near misses (eg, aviation, nuclear energy) to help design better systems for improving safety.2 The authors focus on near misses, incentivising reporting, maintaining confidentiality and a systems approach to error analysis. An AHRQ evidence report on making healthcare safer reiterates that reporting of adverse events and near misses is still an important aspect of use for institutional quality improvement, despite the challenge of how much variation there is among institutions.3
Despite the value of near-miss reporting in other industries, near-miss reporting has not been a well described part of traditional hospital incident reporting systems. Examples of methods to increase near-miss reporting include the institution of a close call reporting system at the Veterans Administration hospital in Martinsburg, West Virginia, the use of safety mailboxes in the paediatric intensive care unit at Primary Children's Hospital in Salt Lake City, and a web-based incident reporting system by the College of Nursing at Columbia University in New York City.4–6 None of this work has been specific to the emergency department (ED) setting, where the pace of work and frequent interruptions are likely to interfere with voluntary reporting of safety events.
The Pediatric Emergency Care Applied Research Network (PECARN) was created in 2001 by the Maternal Child Health Bureau through the Emergency Medical Services for Children (EMSC) Programme, to address limitations of single-site paediatric emergency research. PECARN investigators began work on patient safety in 2006. The initial work used the medical literature and expert consensus to define incident reporting definitions including types, subtypes of injury, severity of harm and contributing factors leading to the event. This led to work describing the state of a ‘culture of safety’, the ED characteristics and the incident reporting systems in place at each site.7 Later work honed in on medication events reported across the network.8 Laboratory error IRs reported harm in 17% of IRs submitted that year, but none serious.9 Our long-term goal was for our preliminary safety work to help lead us to implementation of evidence-based interventions to further enhance safety within our EDs.
In the manuscript defining our methodology, we described near-miss events as events which occur but do not reach the patient, through either chance or by active recovery.10 We defined unsafe conditions as reports which describe a situation that could lead to an adverse event, also often known as latent safety events, where the environment or path to an adverse event is present but something gets in the way prior to reaching the patient.
In this study, our primary objective was to characterise paediatric ED near-miss events and unsafe conditions reported in hospital safety reporting systems in participating PECARN institutions. A secondary aim was to assess the contributing factors, particularly human factor elements and their importance to these events.
Methods
This is a preplanned secondary analysis of a 1-year observational study within PECARN conducted from July 2007 to June 2008, including 18 network EDs. Descriptions of the PECARN EDs have been previously reported.11 All EDs submitted de-identified ED-related IRs to the PECARN data coordinating centre (DCC). IRs were collected using site-specific incident reporting systems. Each participating site had approval of their institutional human subjects’ committee and approval of the legal department for IRs to be electronically transferred to the DCC.
After receipt of the de-identified reports, the DCC catalogued the reports and provided them in electronic format for review by the study team. The study team reviewed either Excel spreadsheets or PDF documents of scanned paper reports, depending on the native format submitted by each site. A unique site identifier was assigned for each site and was masked for the reviewers. The study team created a comprehensive manual of operations to describe the process of review, analysis and definitions of safety events, and used an established taxonomy.10 ,12 IRs were excluded for patients aged 18 years or older, if the incident did not occur while the patient was under ED care or when an incident did not involve the patient or someone accompanying the patient. Each IR was reviewed by two investigators (independent primary and secondary reviewers); differences in interpretation were reviewed by monthly phone calls to achieve consensus.
All IRs were categorised as described in previous work using the nomenclature developed by six paediatric emergency medicine physicians, through review of the current evidence review, and with input from an expert in safety taxonomy (see online supplementary appendix 1).10 ,12 Only two levels of harm were included: unsafe condition—a potentially unsafe situation that could contribute to an adverse event but was not actually associated with a safety event, and near-miss event—an event that occurred but did not reach the patient. The latter could be classified as not reaching the patient by chance alone or due to active recovery efforts by staff.10 Contributing factors were categorised into environmental, equipment, human, information technology, patient or guardian or system factors. If a specific incident involving two patients or two distinct events occurred, we analysed each separately.
Descriptive statistics were used to present the frequency and proportion of types, staff involved and contributing factors. Data was analysed using SAS software, V.9.3, of the SAS system for Windows (copyright 2002–2010 SAS Institute, Inc, Cary, North Carolina, USA) and R software (V.2.13.2), R Foundation for Statistical Computing, Vienna, Austria. After analysis of the results in the IR tables, the investigator (RR) performed a descriptive review of 20% of the IRs to have a better understanding of the specific details reported within the types of near-miss and unsafe conditions. Two sites were chosen (blinded) and 20 consecutive IRs were reviewed from each. Subsequently, two common types of IRs (Medication and Process-related) were selected and 5–10 IRs from each of the contributing sites were reviewed for qualitative details.
Results
From July 2007 to June 2008, 487 reports of near misses and unsafe conditions entered from 18 contributing hospital EDs were analysed for this study (figure 1). This represents 15.7% of all IRs.
Incident reports in PECARN participants (2007–2008). PECARN, Pediatric Emergency Care Applied Research Network.
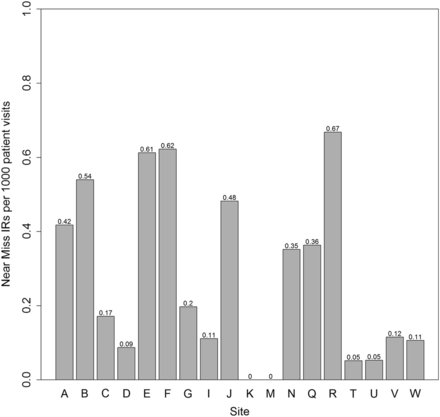
Figures 2 and 3 depict the rates for each of near misses and unsafe condition for all 18 hospitals, expressed as reports per 1000 patients. The rate of reporting for unsafe conditions and near misses ranged from 0.0 to 1.43 per 1000 ED visits among the 18 hospitals. There were two sites with no IRs classified as near misses (K, M) or unsafe conditions (F, T) by the principal investigators and several sites with very low rates. Figure 4 shows the overall monthly rates per 1000 visits of IRs of near misses and unsafe conditions, which did not demonstrate substantial change over the year studied, despite an overall increase of all IRs in the 18 reporting EDs.
Number of near miss incident reports (IRs) per 1000 patient visits by site.
Unsafe condition incident reports (IRs) per 1000 patient visits by site.
Rates of near miss and unsafe condition incident reports/1000 patient visits
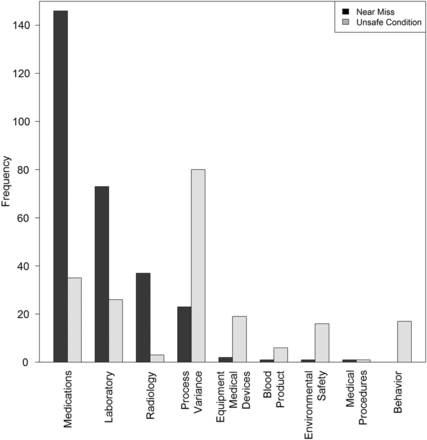
Figure 5 depicts the numbers of IRs from all reporting sites by type or category and separate bars for near miss and unsafe conditions. Medication near misses predominate, followed closely by laboratory, radiology and process-related IRs. Many of these were entered in the IR reporting system by staff outside of the ED. Within IRs reported as unsafe conditions, process-related IRs predominated with a modest number of the other categories. There were very few IRs categorised as near miss or unsafe conditions regarding ED use of blood products or specific medical procedures.
Graph of the near miss and unsafe condition incident reports by category reported
There were 461 of 487 IRs (94.7%) where a contributing factor could be identified by the investigators (table 1). The investigators elicited more than one contributing factor in some IRs; thus the totals are greater than 100%. Human factors issues were listed as contributing to 87% of IRs, followed by equipment in 11%. Other contributing issues were each associated with 5% or less of the IRs. In more than half of IRs in which a parent or visitor is noted as contributing (12/17 reports), the reports were related to parental behaviour perceived as a set-up for unsafe conditions, in which staff perceived the risk of escalation or potential harm to the ED staff.
Contributing factors in near misses and unsafe conditions
Tables 2 and 3 demonstrate the human factors issues reported in near miss and unsafe condition reports.
Human factors issues identified for near miss IRs (N=258)
Human factors issues identified subtypes for unsafe condition IRs (N=141)
Contributing factors
We identified staff non-compliance with an established procedure as a contributing factor in almost 2/3 (n=162) of reports involving near-miss. Most lacked specific details, except for 21 (8.1%) reports where a wrong or no ID was used for patient identification. Non-compliance with an established procedure was the most frequently reported contributing factor in IRs that had unsafe conditions, with 103 (73%) communication skills and clinical judgment next in frequency. Approximately 35% of near miss events were related to one of three issues—mathematical calculations, clinical judgment and communication/interpersonal skills. Of the 38 IRs that involved communications/interpersonal skills, 22/258 (8.5%) noted handoff issues between the ED and other services, and 7/258 (2.7%) were handoffs within the ED. Fatigue, stress or distractions contributed to 2% of reported near miss IRs. In unsafe conditions, handoffs between the ED and other services was indicated in 24/141 (17%) and handoffs in the ED in 6/141 (4.3%).
Results of the descriptive in-depth review of selected incident reports included 81 (17.5%) of the IRs reviewed by the PI, primarily medication-related events (33/181 IRs), laboratory-related issues (23/99) and process variance events (15/103 IRs). Medication and process-related report selection by the PI were performed when these types described latent safety conditions. Events reported included pharmacy sending the intravenous form of the medication when ordered orally, tuberculin skin test at five times the dose, intravenous fluids or medication in the wrong bin in the pharmacy dispenser and sound-alike errors (ie, Propranolol in Promethazine drawer). Process variance issues reported included leaving a child with significant behavioural issues alone inappropriately, contaminated equipment left in ED where it could be mistakenly reused, ID band not on patient on admission to the floor, paperwork incomplete for blood transfusion and incorrect isolation precautions at bedside.
Discussion
This study provides the background from a large paediatric emergency network on how often formal hospital reporting systems received ED-specific data about unsafe conditions and near-miss safety events. We believe that these baseline data reflect several important safety-related factors. First, hospitals during this window of time had highly variable rates of reporting of near-misses and unsafe conditions. This is likely multifactorial, including varying complexity of incident reporting systems and different degrees of emphasis on safety reporting by provider type. Sites with culture of reporting near-misses and unsafe conditions do have potential of gaining better understanding of latent safety issues. Second, the top four reported areas likely reflect real importance to the safety of ED patients—medication issues, laboratory and radiology potential errors and the perception that many providers do not follow approved policy and procedures. These all can lead to harm within emergency care.
Non-compliance with established procedures was by far the largest contributing factor to near-miss events and could represent potential inappropriate and unsafe staff work around. Patient misidentification was the most common identified human factor error. Dosing calculations (ie, medication safety issue), clinical judgment (ie, effective treatment initiated) and handoffs/communications were also identified as common human factors issues that undermine patient safety. Though fatigue and staffing shortage may be important ED environmental factors related to safety, these were infrequently represented in this data. It is likely that ED clinicians, accustomed to fatigue and staffing shortages, may not recognise the contribution of these factors to safety events and thus, these may go unreported.
The reporting of near-miss and unsafe conditions is considered an important component of hospital-based safety initiatives in the report To Err is Human.1 The expert opinion of the IOM supports the development and reporting of data on near-misses and less serious injuries as a key component to improving healthcare across the spectrum of our hospital environment. The pyramid, figure 6, from the IOM report, depicts internal reporting of near-miss events as critical to system improvement with the less common, but preventable, serious events being considered for more generalisable or public reporting. Importantly, many of the potential errors reported in this study also occurred in patients with more severe levels of harm, as reported previously.7 For example, 10-fold medication overdoses or pounds-versus-kilogram medication errors can present as near-misses or as real events with severe harm. Thus, unsafe conditions and near-miss events can provide an opportunity to study and redesign medication delivery processes before events actually reach patients. This is consistent with the Columbia University web-based reporting system.5 When designed to incorporate near-miss and unsafe conditions, clinical IR data sets can help to identify latent safety issues that improvement methodologists can use to implement systems improvements to prevent errors from occurring or reaching the patient.5
Hierarchy of reporting
Scott et al13 described work carried out in Oregon Health and Science University to increase residents’ and physicians’ involvement and reporting of IRs. A small financial incentive was linked to the reaching of the goal of increasing the physician's submission of IRs from a preintervention level of 1.6–5%. After a run-in period, the outcome of the work was a 5.6-fold increase to 9% of IRs being reported by residents. This led to a significant change in processes that helped reduce delays in patient care through both the implementation of effective communication and improved patient–provider partnership. One important barrier the investigators noted they had to overcome was the concern that reporting would lead to individual blame. It is known that staff may have concerns about being treated in a negative way ‘for reporting or discussing medication errors’ when devising the ED reporting system.14
In the industrial world, measurements of near misses and unsafe conditions are not only critical to the culture of safety, but also to the unmasking of latent safety threats. The 2006 IOM report on Performance Measurement emphasises that the development of meaningful and reproducible measures are key to improving the safety of our healthcare systems.15 Teams can use the information to help improve systems’ issues, identify and mitigate unsafe conditions and make the ED a safer place for patients. In the ED setting, there has been success in improving near-miss reporting by instituting patient safety walk rounds, in which senior physicians and nurses discuss safety concerns with front-line staff several times a month, during all shifts and days of the week. These walk rounds help change the culture from blame to praise for identifying and reporting safety concerns.16 Additional improvement work by Mutter17 used reporting of near misses in medication safety as a method to help change the culture away from punitive blame culture towards reporter heroism. Camargo18 found that the safety climate at 62 academic general EDs was associated with an increase in intercepted near misses for myocardial infarction, joint dislocation and asthma in a representative sample of adults. Hospitals have also found that reporting of near misses can help uncover how events were prevented from reaching patients.19 Conlon et al reported on work carried out adapting the principles of a high-reliability organisation and a PEERS (potential error and event reporting system) to lead an effort to rid their system of harmful events. They demonstrated reduced rates of safety issues and a considerable drop in patients mortality.20 Specific to the ED would be Conlon's finding that availability of expertise, a well-designed environment for novice providers and realistic expectations around decision-making are themes that can be used to improve system-level issues in hospitals in high-risk environments such as EDs. Finally, the reporting of unintended events, even without harm, can give us a better understanding of how human and institutional factors contribute to patient safety.21
Overall, within the network, there are increased reporting of latent safety threats since 2008. Data from incident reports have been used to drive systems changes to improve patient safety. Based on review of these incident reports, some hospitals in our network have modified their processes for recording patient weights, for delivering medications to the bedside, and for labelling laboratory specimens. The multi-institutional nature of this study has benefited patients by establishing a learning organisation through shared understanding of safety at other hospitals. The investigation of near-miss events and unsafe conditions has been particularly valuable because these are not only more common than serious safety events, but also provide useful insight into factors that may lead to serious events.
Limitations
The use of IRs to describe near misses and unsafe conditions is clearly challenging, as it is known that voluntary IR systems under-represent the safety events that occur within a healthcare microsystem such as the ED. It is also known that institution reporting rates vary considerably.10 We understand that the use of IR systems is affected by local reporting culture and may not represent the true safety threats that are common in the ED environment. Although incident reports are available to be completed by any hospital staff, there may be inherent provider-specific biases related to the reporting of safety threats. Generally, non-physician providers, including nurses, were more operatively involved in the use of the IR system. In our review of all IRs, we did see patterns where certain types of IRs could be perceived as culture of staff ‘write ups’, rather than direct importance to patients. This was not found in the detailed review of this subset of IRs. Lastly, IRs often lack the granular, qualitative details of the event or the outcomes/interventions related to the incident. Comprehensive review of the circumstances surrounding near miss and unsafe conditions would be helpful when making safety-related recommendations and process improvements.
Conclusions
The reporting of near miss and unsafe conditions in paediatric EDs has helped us understand factors across institutions that could lead to healthcare system changes that focus on improving the culture of safety and reducing risk for patients. The most important latent safety issues reported in our study include medication safety, process-related issues including handoffs and laboratory errors. Importantly, literature would support that inherent in the work is the development of an understanding of how near misses and unsafe conditions can be used for staff to institute the reliable tools that mitigate the risk to patients of being reached by an adverse event.
Future work
Our PECARN safety work group has continued to work to understand and improve patient safety, including seeking to understand the important relationship to causation. We are developing measures and interventions that can be tested at sites and shared across emergency settings. Such future projects will evaluate the relationship between specific safety practices used in the emergency department, such as the use of electronic health records, ED-based pharmacy interventions and the streamlining of the handoff process, and how these may contribute to error reduction.
Acknowledgments
Participating centres and site investigators are listed in alphabetical order: Children's Hospital, Boston (L Nigrovic); Children's Hospital of Buffalo (K Lillis); Children's Hospital of Michigan (P Mahajan); Children's Hospital of New York Presbyterian (M Sonnett); Children's Hospital of Philadelphia (K Shaw); Children's Memorial Hospital (E Powell); Children's National Medical Center (K Brown); Cincinnati Children's Hospital Medical Center (R Ruddy); DeVos Children's Hospital (J Hoyle); Hurley Medical Center (D Borgialli); Jacobi Medical Center (Y Atherly-John); Medical College of Wisconsin/Children's Hospital of Wisconsin (M Gorelick); University of California Davis Medical Center (E Andrada); University of Michigan (R Stanley); University of Rochester (G Conners); University of Utah/Primary Children's Medical Center (C Pruitt); Washington University/St. Louis Children's Hospital (D Jaffe); and University of Maryland (R Lichenstein).
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1 - Online supplement
Footnotes
Collaborators The Pediatric Emergency Care Applied Research Network Steering Committee members are as follows: P Dayan, Chair; E Alpern, L Bajaj, K Brown, D Borgialli, JMC, JM Dean, M Gorelick, D Jaffe, N Kuppermann, M Kwok, RL, K Lillis, PVM, D Monroe, L Nigrovic, E Powell, A Rogers, RMR, R Stanley, M Tunik. MCHB/EMSC liaisons are as follows: D Kavanaugh and H Park. Data Management and Coordinating Center (DCC) is composed of the following members: JM Dean, HLG, R Holubkov, A Donaldson, C Olson, S Zuspan and R Enriquez. Feasibility and Budget Subcommittee (FABS) members are as follows: K Brown and S Goldfarb, cochairs; E Crain, E Kim, S Krug, D Monroe, D Nelson, M Berlyant and S Zuspan. Grants and Publications Subcommittee (GAPS) members are as follows: L Bajaj, chair; E Alpern, KJO'Connell, A Cruz, L Babcock, R Mistry, T Chun, W Schalick, O Badaki, F Moler, RMR, L Tzimenatos, A Webster, M Gorelick, J Zorc, Protocol Review and Development Subcommittee (PRADS) members are as follows: L Nigrovic, Chair; JMC, P Dayan, JM Dean, R Holubkov, D Jaffe, E Powell, KNS, R Stanley, M Tunik. Quality Assurance Subcommittee (QAS) members are as follows: K Lillis, chair; E Alessandrini, SB, R Enriquez, RL, PVM, R McDuffie, RMR, B Thomas, and J Wade.
Contributors RMR, JMC and KNS contributed to the conception of the work, acquisition of data and interpretation of results. They were the key in drafting and revising the work. PVM, KJO'C, SB and RL contributed to the design of the work, and to data acquisition and for critical revisions of the content. TF completed much of the analysis and contributed to the interpretation of the data. He was critically involved in the drafts and revisions. HLG was involved with design of the work, data acquisition and revisions of the drafts. Each of the authors gave final approval of the version published and agree to be accountable for all aspects of the work as required.
Funding This project is supported in part by the Health Resources and Services Administration (HRSA), Maternal and Child Health Bureau (MCHB), Emergency Medical Services for Children (EMSC) Programme through the Pediatric Emergency Care Applied Research Network (PECARN). PECARN is supported through the following cooperative agreements: U03MC00001, U03MC00003, U03MC00006, U03MC00007, and U03MC00008, U03MC22684 U03MC22685.
Competing interests None declared.
Ethics approval CCHMC IRB (and IRBs from each of the participating sites).
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Data agreements for this study were not approved for Incident Reports to be in a public use data set as is standard in all other PECARN projects.