Article Text
Abstract
Purpose To quantify the impact of bariatric surgery on cardiovascular (CV) risk factors, and on cardiac structure and function.
Data sources Three major databases (PubMed, Medline and Cochrane) were searched for original studies written in English.
Study selection Original articles reporting CV risk factors or non-invasive imaging parameters for patients undergoing bariatric surgery, from January 1950 to June 2012.
Data extraction Data extraction from selected studies was based on protocol-defined criteria that included study design, methods, patient characteristics, surgical procedures, weight loss, changes in CV risk factors, cardiac structure and cardiac function postoperatively.
Data synthesis 73 CV risk factor studies involving 19 543 subjects were included (mean age 42 years, 76% female). Baseline prevalence of hypertension, diabetes and hyperlipidaemia were 44%, 24%, and 44%, respectively. Mean follow-up was 57.8 months (range 3–176) and average excess weight loss was 54% (range 16–87%). Postoperative resolution/improvement of hypertension occurred in 63% of subjects, of diabetes in 73% and of hyperlipidaemia in 65%. Echocardiographic data from 713 subjects demonstrated statistically significant improvements in left ventricular mass, E/A ratio, and isovolumic relaxation time postoperatively.
Limitations Diagnostic criteria, CV risk factor reporting, and imaging parameters were not uniform across all studies. Study groups were heterogeneous in their demographics, operative technique and follow-up period.
Conclusions This systematic review highlights the benefits of bariatric surgery in reducing risk factors for CV disease. There is also evidence for left ventricular hypertrophy regression and improved diastolic function. These observations provide further evidence that bariatric surgery enhances future CV health for obese individuals.
- Basic science
- oxidative stress
- cardiac function
- systolic dysfunction
- heart failure
- heart failure treatment
- metabolic medicine
- diabetic heart disease
- cardiomyopathy
- valvular disease
- cardiac surgery
- heart transplant
- artificial heart
Statistics from Altmetric.com
- Basic science
- oxidative stress
- cardiac function
- systolic dysfunction
- heart failure
- heart failure treatment
- metabolic medicine
- diabetic heart disease
- cardiomyopathy
- valvular disease
- cardiac surgery
- heart transplant
- artificial heart
Introduction: obesity and the heart
Obesity is one of the greatest public health challenges of current times, with over 2.6 million people dying annually as a result of being overweight or obese.1 The ‘overweight’ and ‘obese’ states are defined in terms of body mass index (BMI), with overweight being 25–30 kg/m2, class I obesity 30–35 kg/m2, class II obesity 35–40 kg/m2 and class III obesity >40 kg/m2. Obesity is associated with an increased metabolic burden of hypertension, dyslipidemia and diabetes mellitus, which translates into elevated rates of cardiovascular disease (CVD) and heart failure (HF).2 ,3 There is growing evidence that obesity-associated coronary artery disease and myocardial dysfunction may be a direct consequence of the excess adipose tissue.4 Individuals with excessive and dysfunctional visceral adiposity tend to demonstrate a proinflammatory state, insulin resistance, endothelial dysfunction, and development of myocardial hypertrophy, all of which may be promoted by altered levels of ‘adipokines’ such as adiponectin, resistin and leptin.
Weight loss—achieved by any means—improves CV risk, CV outcomes and probably also mortality.5 Diet and exercise always play a role in obesity management, but lifestyle interventions alone do not achieve durable weight loss in the majority of patients.6 Bariatric surgery has emerged as the most effective and durable strategy for successful weight loss in obese individuals. The bariatric procedures incorporating malabsorptive functions, such as Roux-en-Y gastric bypass (RYGB) and biliopancreatic diversion (BPD), are associated with the greatest weight loss and metabolic benefits. Patients undergoing malabsorptive surgeries demonstrate more marked restoration towards normal serum profiles of adipokines and gut hormones, and it is postulated that this return towards non-obese metabolic function contributes to the positive effects of bariatric surgery on CV risk factors such as diabetes. The biochemical responses to bariatric surgery are also believed to impact on the heart structurally, for example, via leptin signalling.7–9
Current bariatric surgery criteria are outlined in box 1, although surgical indications continue to evolve. Weight loss after surgery is typically expressed in terms of ‘excess weight’, which refers to the difference between the actual and ideal weights for an individual. The primary goal of bariatric procedures is weight loss, and while the various procedures achieve this to different extents, the overall percentage of excess weight loss (EWL) is reported to be as high as 47–70%.12 Perioperative morbidity and mortality has fallen considerably with widespread adoption of laparoscopic approaches, and the most recent Longitudinal Assessment of Bariatric Surgery reports 30-day mortality of 0.3%.13
Current criteria for consideration of bariatric surgery
Unsuccessful weight loss with dietary and exercise interventions, and:
The purpose of this systematic review was to accurately gauge the impact of bariatric surgery on CV risk factor reduction, using the most recently published data to expand on our previous observations.14 Additionally, we sought to quantify the impact of bariatric surgery on cardiac structure and function, both in obese individuals without known CVD and in those with a systolic HF diagnosis.
Methods
The present review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.15 We conducted a comprehensive review of all studies published in English containing data on CV risk factors, risk reduction, HF outcomes or structural cardiac changes in obese patients after any form of bariatric surgery. We performed an electronic search in three major databases (PubMed, Medline and the Cochrane library) for relevant original studies published from 1st January 1950 to 1st June 2012. The following search terms were employed.
-
To identify studies evaluating CV risk factor changes with bariatric surgery: bariatric surgery (MeSH) OR obesity/surgery (MeSH) OR gastric bypass (MeSH) OR gastroplasty (MeSH) OR BPD (MeSH) OR jejunoileal bypass (MeSH) OR gastric banding OR duodenal switch AND (CV diseases (MeSH) AND outcome assessment (Health Care) (MeSH) OR outcomes.
-
Three searches were performed to identify studies which evaluated HF outcomes and/or structural cardiac changes after bariatric surgery: bariatric surgery (MeSH) OR obesity/surgery (MeSH) OR gastric bypass (MeSH) OR gastroplasty (MeSH) OR BPD (MeSH) OR jejunoileal bypass (MeSH) OR gastric banding OR duodenal switch OR gastric sleeve AND:
-
Heart diseases (MeSH).
-
Cardiac output, low (MeSH) OR cardiomegaly (MeSH) OR cardiomyopathies (MeSH) OR HF (MeSH) OR ventricular dysfunction (MeSH).
-
Cardiac imaging techniques (MeSH).
-
In addition to the primary electronic search we reviewed the ‘related citations’ in PubMed, and manually reviewed the bibliographies of selected articles and reviews published in the last 10 years. Studies were included if they reported on clinical outcomes (such as hypertension, hyperlipidaemia and diabetes), objective measures health (laboratory data, echocardiograms, cardiac MRI) or CV risk estimates. Exclusion criteria were case reports, review articles and studies reporting on surgical technique or weight loss only as outcomes. The minimum number of participants was set at 50, with lower thresholds of 30 for the rare studies randomising participants to surgical versus non-surgical management and 10 for studies of cardiac structure/function. Publications involving duplicate patient populations were identified and grouped together; only the most recent or parent study was included to avoid double counting subjects.
Data extraction from selected studies was based on strictly defined criteria. Basic descriptive statistics were used to summarise patient and study characteristics, reported weight loss and CV risk factor data. Outcomes collected for CV risk reduction included resolution or improvement in hypertension, hyperlipidaemia and diabetes, and changes in blood pressure, lipid profile, C reactive protein (CRP) and Framingham Risk Score (FRS). Other efficacy outcomes collected for the risk factor studies included %EWL. Where postoperative BMI was stated instead of EWL, the %EWL was calculated as follows, assuming an ‘ideal weight’ of 25 kg/m2: (BMI baseline−BMI postoperatively)/(BMI baseline−25)×100.
Outcomes were assessed at the longest time-point for which data were available for at least 50% of the initial population. In studies with a control group, baseline and follow-up values were extracted for the surgical cohort only. Where studies reported their results by gender, ethnicity or procedure type we calculated the overall results using the proportional mix of genders (or other variable) and individual scores. CV risk reduction indicators were expressed as the percentage of patients who had resolution or improvement of hypertension, hyperlipidaemia and diabetes as determined by clinical or laboratory measurements, or by changes in postoperative medication requirements for each comorbidity. If both resolution and improvement were presented separately, only the percentage achieving resolution was extracted. The denominator used for percentage resolution was the initial number of participants, rather than only the participants completing follow-up, to provide a conservative estimate.
All mean parameters calculated were weighted means; the denominator varied according to the parameter reported. Pooled analysis of the presurgical and postsurgical echocardiographic parameters was performed using fixed effects meta-analysis, utilising Stata V.12.0 software (Stata Corporation, College Station, Texas, USA). In cases of significant heterogeneity (I2>50%, p<0.05) a random effects analysis was used. Meta-regression analysis was used to determine the impact of changes in blood pressure upon left ventricular mass (LVM). Publication biases were assessed using funnel plot method as well as Egger regression asymmetry testing.16 The three small trials randomising participants to surgical versus non-surgical management of diabetes were initially analysed separately due to their distinct inclusion criteria, but due to their similar effect estimates as the non-randomised studies, the numbers presented represent the full pool of data.
Results
After the initial screening of titles and abstracts regarding CV risk factors (n=1119), 971 were excluded and 148 studies were reviewed to determine whether they met the inclusion criteria (figure 1A). Of these, 54 were excluded at this stage of the review because they did not meet inclusion criteria. Upon review of the remaining 94 publications in full, a further eight studies were identified from bibliographies and related citations which were deemed suitable for inclusion. Of the 102 primary studies, 73 contained sufficient detail to merit inclusion in the extractable and analysable dataset.
We also searched for articles reporting on non-invasive imaging parameters of cardiac structure and function before and after surgical weight loss (figure 1B). After exclusion of kin studies, one study that did not separately report the surgical subjects (as opposed to lifestyle modifications only),9 two without specified absolute values for imaging parameters,8 ,17 and one that focused on the right ventricle,18 there were 16 studies available for pooled analysis. The two papers by Karason et al included the same cohort but reported differing imaging parameters.
(A) Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) flowchart for studies reporting on cardiovascular risk factors. (B) PRISMA flowchart for studies reporting on cardiac structure and function.
We also identified a total of 10 studies that reported bariatric surgery outcomes for obese patients with pre-existing HF. Four of these were case reports and were excluded. One study19 was excluded due to its overlapping date range at the same institution as Ramani et al. Only four of the remaining studies reported left ventricular systolic function before surgery and post surgery; Alpert et al in 1985 and 1997 reported fractional shortening rather than left ventricular ejection fraction (LVEF) and therefore could not be included in the pooled analysis.
Bariatric surgery and cardiovascular risk
The 58 traditional CV risk factor studies, 12 studies reporting on novel CV risk factors (such as inflammatory markers or risk scores) and three studies randomising patients with diabetes to bariatric surgery versus optimal medical therapy yielded 19 543 patients in total (table 1).93 The majority of studies originated from USA and Europe, with Asia becoming more frequently represented in recent years. Data from three studies published by the Swedish Obese Subjects Study Scientific Group were merged to ensure that the patient cohort was counted only once.
Summary of cardiovascular disease (CVD) studies included in review
The baseline mean age of the patients was 41.7 years (range of mean ages 27–54), 76% of the population was female and the mean BMI (kg/m2) was 47.1 (range of mean BMIs reported 30–60). Mean follow-up was 57.8 months (range 3 to 176.4) and average EWL was 54.2% (range 16–87). The baseline prevalence of obesity-related comorbidities that increase CV risk was: hypertension 44.4%, diabetes 24.0%, and hyperlipidaemia 43.6% (table 2). Malabsorptive or bypass procedures were most commonly performed with RYGB and BPD accounting for 57% of cases, compared to gastric banding in 27%.
Results of cardiovascular disease (CVD) studies
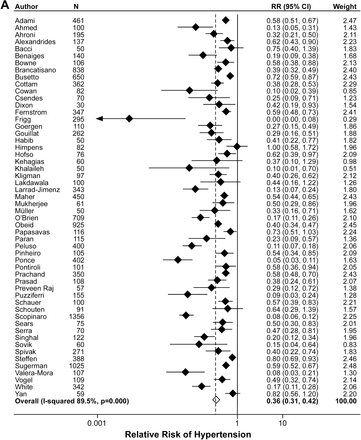
As reported in Table 2, and graphically displayed in figure 2A–C, a substantial proportion of study subjects showed resolution or improvement of their baseline hypertension (62.5%), diabetes (73.2%) and hyperlipidaemia (65.2%) at follow-up. We did identify significant publication bias in the studies reporting on all three of these comorbidities (figure 3, hypertension p<0.001 panel A, diabetes p<0.001 panel B, hyperlipidaemia p=0.012 panel C). After using the ‘fill and trim’ method of correction for the meta-publication bias,94 corrected effect estimates were 0.36 (0.31 to 0.42) for hypertension, 0.26 (0.21 to 0.31) for diabetes, and 0.34 (0.28 to 0.40) for hyperlipidaemia. These values demonstrate statistical significance for the postsurgical improvements in all three comorbidities.
(A) Forest plot for standardised mean difference for hypertension. (B) Forest plot for standardised mean difference for diabetes. (C) Forest plot for standardised mean difference for hyperlipidaemia.
(A) Funnel plot assessing publication bias for hypertension studies. (B) Funnel plot assessing publication bias for diabetes studies. (C) Funnel plot assessing publication bias for hyperlipidaemia studies.
As a result of the risk factor improvements, there was a reduction in 10-year coronary heart disease FRS, pooled from five studies, from 5.9% to 3.3%. We also calculated the 10-year global CVDFRS based on weighted mean data to represent average patients in the overall pooled cohort. A female without baseline CVD, diabetes or smoking, who is taking antihypertensive medication both presurgery and postsurgery, and whose age increases over the mean 58-month follow-up period will move from a 10-year global risk of 8.6% to 3.9%. A male with no CVD or smoking, but whose diabetes and need for antihypertensive medications resolves post-bariatric surgery will move from a global risk of 18.4% to 4.7%.
Of the 12 studies reporting the effect of bariatric surgery on novel and emerging risk factors for CVD, the most frequently reported biomarker was CRP which was described preoperatively and postoperatively in eight studies (1157 subjects). Mean CRP levels were elevated at baseline in obese subjects (9.1 mg/l) but decreased by 73% (to 2.5 mg/l) after surgically-induced weight loss. CRP levels have been shown to correlate with 8-year CV event and death rates in initially healthy females.95
Bariatric surgery and myocardial structure
The parameters reported across the 16 cardiac imaging publications varied considerably between studies, but LVEF (%), LVM (g), LVM indexed to height (g/m2.7), left atrial diameter (mm), left ventricular diastolic dimension (mm), E/A ratio from the mitral inflow Doppler and isovolumic relaxation time (IVRT, mm) were the most frequently reported values. There were a total of 713 subjects, and RYGB was the most popular procedure performed (70% of surgeries reported). As per table 3, mean age was 39.4 years, 73% of subjects were female and the average follow-up period to postoperative imaging was 20.2 months. Mean BMI was 48.7 kg/m2 preoperatively and 31.6 kg/m2 at the latest point of follow-up. Most of these studies had a prospective cohort design, but only a minority had matched non-surgical control groups and none were randomised.
Summary of cardiac structure and function studies included in review
LVM showed a significant postoperative reduction (figure 4B,C, table 4), with a standardised mean difference of 0.69 (95% CI 0.52 to 0.85). We also analysed pooled data for LVM and systolic BP at the time of pre operative and postoperative imaging, which was available for seven of the eight studies reporting LVM. This demonstrated that the mean difference in systolic BP between preoperative and postoperative echocardiograms did not significantly impact on the reduction in LVM (p=0.18), indicating that left ventricular hypertrophy regression is not simply a function of hypertension improvement. The reduction in LVM was not accompanied by significant postoperative reductions in chamber dimensions; the standardised mean difference (SMDs) for left atrial diameter (217 subjects) and left ventricular diastolic dimension (650 subjects) were non-significant.
Results of cardiac structure and function studies
Our pooled data demonstrated a clear improvement in E/A ratio from 1.28 to 1.48 across 391 subjects (figure 4F, table 4). There was also a significant improvement in IVRT, which is one of the most consistent diastolic abnormalities seen in obese individuals, from 84.0 to 72.9 ms (figure 4G, table 4). LVEF was reported in eight studies, incorporating 471 patients without a HF diagnosis. The mean preoperative LVEF was normal at 63.2% and did not significantly change postoperatively (figure 4A, table 4).
(A) Forest plot for standardised mean difference for LV ejection fraction. (B) Forest plot for standardised mean difference for LV mass. (C) Forest plot for standardised mean difference for LV mass index. (D) Forest plot for standardised mean difference for left atrial diameter. (E) Forest plot for standardised mean difference for LV diastolic diameter. (F) Forest plot for standardised mean difference for E/A ratio. (G) Forest plot for standardised mean difference for isovolumic relaxation time.
Bariatric surgery and HF
There is very limited published data on obese patients with systolic HF who undergo a bariatric procedure. We retrieved four publications reporting preoperative and postoperative values for systolic function in HF cohorts. Data was collated for 21 patients with a mean preoperative LVEF of 31.6% (age 46, 69% female), with the majority of patients undergoing RYGB. BMI fell by an average of 14.6 kg/m2 at follow-up (mean 25 months). The mean postoperative LVEF was 45.5% (table 4); no statistical calculation of significance was made due to the small number of studies. There were two studies reporting length of stay as 3.0 and 3.3 days respectively, which is in line with the expected hospital stays for unselected bariatric surgery patients.111 ,112
However, particularly significant limitations exist within these small HF studies, including the potential for bias in echocardiographic readers who are not blinded to surgical status. Caution must also be exercised regarding the natural history of the systolic function in non-surgical control patients with HF: Ramani et al studied a control group that showed no change in LVEF and a deterioration in New York Heart Association symptom class over the same follow-up period as the surgical subjects, but Garza et al observed that patients with LVEF ≤50% experienced LVEF improvements regardless of whether or not they were in the surgical intervention group.
Limitations
Our analysis of CV risk factors carries many limitations inherent to the component studies. First, the criteria for ‘improvement’ or ‘resolution’ of comorbidities varied greatly between publications. Most utilised objective laboratory data—although presumably with variations in laboratory techniques and diagnostic thresholds—but other studies based their diagnoses on patient reports of comorbidities or medication usage. The second major limitation of this, and indeed most studies of bariatric surgery outcomes, is the high attrition rate of patients available for follow-up. We only included studies where >50% of the initial cohort remained, but loss to follow-up may still have skewed results. The follow-up periods were also markedly variable, ranging from 3 months to 14.7 years. Third, the absence of a standardised format for reporting outcomes in bariatric surgery trials limited our ability to perform pooled analyses. There is a clear need for trials in the field to consistently report actual weight lost and %EWL, along with baseline prevalence and subsequent improvement in metabolic comorbidities, using biochemical data and medication requirement for verification.
Several study design limitations common to both the CV risk and cardiac structure studies should also be considered. Very few bariatric surgery trials have randomised subjects to operative versus non-operative management, and hence the self-selected populations of patients who pursue surgery may not be generalisable to the full spectrum of obese individuals. Studies employed heterogeneous bariatric procedures, and among RYGB studies there were variations with respect to pouch size and lengths of the Roux limb, all of which could affect comorbidity remission. Of note 68% of Swedish Obese Subjects (SOS) participants received vertical banded gastroplasty, a restrictive procedure that is no longer performed in the US; RYGB was the most common procedure in North America as of 2008.113 We were also unable to control for variations in medication dosages that are likely to have occurred in the postoperative period.
Specific to the studies of cardiac structure and function are potential variances in methods of measurements of echocardiographic parameters, particularly in the calculation of LVM, where several equations exist. Differing echocardiogram machines, measuring techniques and levels of interpreter skill may also limit data validity.
Discussion
This systematic review provides useful additional information for the physician who is advising a patient with obesity on their future risk of adverse CV outcomes. The pooled analysis of 19 543 bariatric surgery subjects lends further weight to the existing literature supporting a marked reduction in CV risk factors and calculated risk scores in the short to medium postoperative period. These observations have elevated bariatric surgery beyond the realms of a cosmetic procedure and into the spectrum of interventions demonstrating efficacy in preventing CV events. The magnitude of effect on CV risk factors is impressive, and to date no pharmacological therapy for weight management or diabetes has shown a comparable effect over these short time periods.
The next step should be to move from risk factors or scores to routinely collecting data on long-term (>10-year) actual CV events, CV mortality and all-cause mortality. Two included trials did report on actual CV events or mortality. Torquati et al reported on 500 RYGB patients who achieved a mean 68% EWL at 1 year and a reduction in 10-year Framingham coronary heart disease event risk from 5.4% to 2.7%.93 The actual rate of postoperative cardiac events observed in this high-risk surgical cohort was as low as 1% at 5 years. The SOS study reported a reduced CV mortality in 2010 surgical patients who were matched to a non-operative control cohort.5 Despite significantly more prevalent smoking and higher baseline weights and blood pressures in the surgical cohort, bariatric surgery was associated with a lower number of total first-time CV events (9.9% vs 11.5% adjusted HR 0.67, p<0.001), fatal myocardial infarctions and total myocardial infarctions, than controls. CV deaths were also reduced, with 1.4% CV mortality in subjects versus 2.4% in controls, adjusted HR 0.47, p=0.002. The mean 14.7-year follow-up represents the longest report of bariatric surgery outcomes to date.
Interestingly, it was the baseline degree of insulin resistance, rather than initial BMI or the amount of weight lost, which was most predictive of CV benefit in the SOS cohort. This observation emphasises the importance of appropriate case selection, as BMI is a poor indicator of the degree of adiposity or metabolic dysfunction. Younger females are the most represented demographic for bariatric surgery (76% female, mean age 41.7 in this analysis), but despite the potential for relief for conditions such as infertility and urinary incontinence,114 ,115 they have a low baseline CV risk and therefore may not attain a significant CV event or mortality benefit from surgery. Bariatric surgery is accompanied by significant perioperative risks, including (for laparoscopic RYGB) a 2% risk of reoperation for haemorrhage, 2% anastomotic leak, 8% anastomotic ulcer, 5% intestinal obstruction, 5% anastomotic stricture and a 0.3% risk of death.13 ,116 The overall absolute mortality benefits described thus far are comparatively modest (1% absolute risk reduction in CV mortality in SOS) so it is imperative that case selection targets individuals whose potential for long-term health gains considerably exceed operative risks.
Four additional large studies reported exclusively on CV events and mortality after bariatric surgery. Christou et al followed 1035 Canadian surgical patients and matched them to 5746 obese controls extracted from a health insurance database.117 Mortality in the surgical cohort was 0.68% at 5 years, inclusive of 0.4% perioperative mortality, as compared to 6.17% for controls (RR 0.11, 95% CI 0.04 to 0.27). A modest survival benefit was also reported by Flum and Dellinger.118 At 4.4 years median follow-up (15.5 years maximum), 11.8% of the 3328 gastric bypass patients had died, compared to 16.3% of the 62 781 non-surgical obese subjects serving as controls. Adams et al compared their cohort of 7925 RYGB patients with a group of severely obese controls drawn from driver's license applicants in Utah, matched on age, gender an BMI.119 At a mean of 7.1 years of follow-up (18 years maximum), all-cause mortality was 40% lower for surgical subjects than controls (adjusted HR 0.60, 95% CI 0.45 to 0.67, p<0.001). The surgical cohort showed lower mortality rates for coronary artery disease (59%, p=0.006), diabetes (92%, p=0.005), and cancer (60%, p=0.001), but rates of death not caused by disease, such as accidents and suicide, were 58% higher (p=0.04).
Conversely, a recent publication by Maciejewski et al suggested an absence of mortality benefit after bariatric surgery. This study retrospectively compared survival data of veterans who had undergone bariatric surgery to a mean of 6.7 years' follow-up, with matched controls who were untreated for obesity.120 The 2-year and 6-year crude mortality rates were significantly lower for surgical subjects (2.2% vs 4.6% and 6.8% vs 15.2%, respectively). However, after Cox regression analyses utilising propensity score matching, no mortality benefit for surgical patients remained. This study may not be generalisable due to its atypical bariatric surgery population (73.9% male, average age 49.5, 1.29% perioperative mortality), but reinforces the scope for characteristics specific to obese patients who undergo surgical intervention, as compared to those who receive medical management, to confound the relationship between bariatric surgery and CV outcomes or mortality. This highlights the importance of prospectively randomising subjects to surgical versus non-surgical obesity management in future studies of bariatric surgery outcomes. Although the studies of Dixon, Schauer and Mingrone only contributed 170 patients—all with baseline diabetes—to our analysis, their randomised methods make their data on risk factor resolution more convincing.
Mortality end-points are of key importance in this field in light of the observation of an ‘obesity paradox’. Patients with higher BMIs have often shown unexpectedly superior survival rates than normal-weight counterparts, for example, in the short-term period after percutaneous coronary intervention.121 ,122 The paradoxical survival relationship in patients with coronary artery disease disappears when survival is correlated to waist circumference, rather than BMI,123 but persists with anthropometric measurements of obesity in HF cohorts. More favourable survival in patients with a greater degree of obesity was recently demonstrated in advanced systolic HF patients stratified by both BMI and waist circumference.124 Analysis of chronic HF subjects in the SOLVD and V-HeFT II trials also revealed excess mortality in participants who lost more than 6% of bodyweight during the study duration.125 In these studies, lower BMIs may simply be a marker of cardiac cachexia and more advanced HF. However this background does present a greater and more urgent need to prospectively determine whether surgical weight loss does truly enhance long-term survival, particularly in those with existing cardiac disease.
This is the largest systematic review to date of myocardial structure and function after surgical weight loss, and is valuable in defining the potential of bariatric surgery to impact the heart. The pooled data demonstrates regression of left ventricular hypertrophy and improved diastolic function in terms of E/A ratio and IVRT in patients without overt cardiac disease, but without accompanying changes in left atrial of left ventricular dimensions. Reduction in LVM has been seen across most studies documenting the cardiac effects of dietary,126 dietary and exercise,127 pharmacological128 or surgical interventions to induce weight loss. Our analysis demonstrated independence from changes in blood pressure, as previously suggested.100 ,129 ,130 This supports the hypothesis that it is not a direct haemodynamic adaptation to weight loss, but perhaps the altered metabolic profile, that results in LV mass regression.7 ,131 ,132
It is wholly possible that the improvements in E/A ratio and IVRT are solely the result of LVM reduction. This dataset cannot confirm whether subjects who experienced the greatest changes in LVM tended to show the most improvement in diastolic function, although this link was suggested in one of the kin studies by Alpert et al.133 Alternatively, the improved diastolic dysfunction could be due to regression of the myocardial fibrosis, that is associated with obesity, rather than reduced LVM per se. It is noteworthy that although our pooled echocardiographic data did not show a decrease in left atrial or left ventricular diameter after bariatric surgery, MRI-measured ventricular end diastolic volume was significantly lower at a year after weight loss (achieved by diet or surgery) in the 30 obese subjects studied by Rider et al. With superior volumetric left atrial measurements instead of simple diameters, a postoperative difference may have been observed. Alternatively, atrial remodelling may truly be absent with surgical weight loss, or occur over a longer time-period than the studies analysed.
There was no effect of surgical weight loss on LVEF, although more sensitive echocardiographic techniques such as mid-wall fractional shortening,108 integrated backscatter134 and 2D speckle tracking-derived strain and strain rate imaging135 ,136 have highlighted the existence of obesity-associated subclinical systolic dysfunction, which improves after bariatric surgery.99 Such techniques may even offer opportunities to detect incipient systolic dysfunction at a preclinical stage and intervene with lifestyle, pharmacological or surgical methods, to prevent overt HF. Obesity is a risk factor for clinical HF, with a prospective Framingham study of 5881 participants demonstrating that the risk of symptomatic HF increases by 5% for men and 7% for women, per unit BMI increase, despite adjustments for CV risk factors.3 ,137 ,138 Case reports have described bariatric surgery as a ‘rescue’ intervention for very obese patients with advanced systolic HF.139 However, due to the limitations described above in interpreting the few HF studies currently available for review, further data is necessary before a judgement can be made on the role of bariatric surgery in this patient group.
Overall, this systematic review clearly illustrates a marked beneficial effect of bariatric surgery not only on future CV risk, but also on the myocardial mass and diastolic function. Bariatric surgery is certainly not without potential morbidity and mortality, but in appropriately selected obese patients—especially those with a high CV risk—surgical weight loss could be lifesaving. Data such as this provides further support for the application and refinement of surgical approaches for the treatment of obesity with a goal to optimising future cardiac health.
References
Footnotes
Disclosures The authors did not receive funding for this work. PRS disclosures include: Ethicon Endo-Surgery: consultant, scientific advisory board member, research support; Remedy MD: board of directors; Stryker Endoscopy: scientific advisory board, educational grant; Bard/Davol: scientific advisory board, consultant; Gore: consultant, educational grant; Baxter: educational grant; Barosense, Surgiquest, Cardinal/Snowden Pencer: scientific advisory board; Covidien: educational grant; Allergan: educational grant; Surgical Excellence LLC: board of directors. No other authors have any financial disclosures. The authors would like to disclose that this systematic review expands upon a preliminary review of this topic published by Heneghan et al in Am J Cardiol 2011.
-
Competing interests None.
-
Provenance and peer review Commissioned; externally peer reviewed.