Article Text
Abstract
Objectives To localise the immunogenic part of infliximab and evaluate the clinical usefulness of measuring antibodies against infliximab fragments.
Design Observational study.
Settings A specialised inflammatory bowel disease (IBD) centre in a tertiary hospital.
Interventions Serum was collected from patients with IBD and controls. Antibodies against whole infliximab (ATI) and against the digested Fc, F(ab′)2 and F(ab′) fragments were measured by a specifically developed ELISA and by western blotting. A separate ELISA was used to determine infliximab levels in serum.
Results 109 serum samples from 62 infliximab-treated patients were tested along with 64 control samples. Anti-F(ab′)2 antibodies were found in 28/42 (67%) samples with positive ATI, all from infliximab-exposed patients. Anti-F(ab′)2 antibodies were also present in 26 of the remaining 67 (39%) samples from exposed patients despite absent ATI. No specific anti-Fc antibodies were detected. Low trough infliximab level and high ATI level was found in 10/12 patients (83%) with complete loss of response to infliximab, but in only 5/14 patients (36%, p=0.02) who regained response to intensified infliximab regimen and in 2/24 patients (8%, p<0.001) in maintained remission while on 5 mg/kg/8 week infliximab treatment. Although Anti-F(ab′)2 antibodies showed similar test characteristics to ATI in patients losing response to infliximab, they were also detected in 61% of patients in maintained remission, thereby limiting their clinical usefulness. No cross reactivity to adalimumab was noted.
Conclusions F(ab′)2 is the immunogenic fragment of infliximab. However, ATI level in serum—combined with measurement of trough infliximab level—is better correlated with the clinical response to infliximab or with its loss.
- Inflammatory bowel disease
- antibodies
- therapy
- immunology
- inflammatory bowel disease
Statistics from Altmetric.com
Significance of this study
What is already known about this subject?
A substantial portion of infliximab-treated patients with inflammatory bowel disease develop antibodies to infliximab (ATI).
Development of ATI has been associated with clinical loss of response in some but not all studies.
There is scant data pertaining to the immunogenic fragment of infliximab.
What are the new findings?
Trough levels of ATI are strongly associated with clinical loss of response.
The immunogenic fragment of infliximab is F(ab′)2.
How might it impact on clinical practice in the foreseeable future?
For clinical purposes, using the intact molecule as an antigen and measuring ATI is more useful than measurement of anti-F(ab′)2 antibodies.
Introduction
Infliximab and adalimumab are monoclonal antibodies against tumour necrosis factor α (TNFα) which have been increasingly used in the recent decade for the treatment of inflammatory bowel disease (IBD) and other disorders. The proven efficacy of these biological agents may be offset by their immunogenic potential, that is their propensity to elicit in the recipient patient an immune reaction against the drug.1 2 Several reports have shown a correlation between the development of antibodies to infliximab (ATI) and reduced or shortened drug efficacy in IBD3–5 and in rheumatoid arthritis patients.6 The presence of ATI was also associated with increased risk for infusion reactions and for delayed hypersensitivity reactions.3 7 In contrast, other studies showed that clinical response correlated with infliximab trough level but not with the presence or absence of ATI.8
In this context, scant data suggest that the immunogenic part of the infliximab molecule lies within the F(ab′) fragment of the antibody.9 However, there have been no studies examining the diagnostic utility of determining the levels of antibodies to infliximab fragments as opposed to assay of ATI directed against the intact molecule. This may be important since different epitopes revealed by the breakdown of infliximab to its fragments may be more readily bound by antibodies in the serum. Conceptually, the detection of this class of antibodies may therefore change the test characteristics vis-à-vis ATI measurement and potentially improve the assay's accuracy and clinical relevance. Therefore, the aim of the present study was to identify the location of the immunogenic component of infliximab in patients with IBD and to determine whether serum levels of antibodies to this specific fragment possess superior clinical utility compared to ATI.
Methods
Patient population and outcome definitions
Patients with IBD attending the Gastroenterology Department of the Sheba Medical Center were studied. We included patients treated by infliximab as well as unexposed IBD patients and additional controls recruited from patients with unrelated disorders and from healthy volunteers. All patients gave written informed consent and the study was approved by the Sheba Medical Center Ethics committee.
For infliximab-treated patients serum was drawn immediately before their scheduled infliximab infusion, whereas for unexposed subjects blood was drawn during a clinic visit. The clinical characteristics of the IBD patients were extracted from their charts.
Complete loss of response was defined as cessation of infliximab therapy due to lack of efficacy within 3 months following the index infusion (when blood was drawn), regardless of whether the patient was switched to another anti-TNFα agent or an alternative immunomodulator, or referred to surgery. Regained response was defined as exacerbation of IBD at any time during infliximab treatment prior to the blood drawing infusion that responded to an increase in infliximab dose or to shortening of infusion interval. Maintained response was a continued clinical remission or clinical response induced by continued infliximab at regular 8 week intervals. Because of the retrospective nature of the study and the lack of routine clinical score recording, the response of patients was derived from the judgement of the treating physicians as documented in the patients' charts. Infliximab trough levels and antibodies to infliximab or its fragments were measured, and the results were correlated with the patient response to infliximab as defined by one of three possible states (response, regain of response or loss of response). When an individual patient had several trough sera available, only the last sample was considered in determining the ATI status for the analysis of its correlation with clinical outcome.
Determination of infliximab levels
Infliximab levels in serum were measured by ELISA as described by Baert et al with some modifications. Briefly, 100 μl of 1:100 diluted serum was added to pre-plated 750 ng/ml TNFα (Peprotech, Rocky Hill, New Jersey, USA) and incubated for 90 min. Following washing, horseradish peroxidase (HRP) labelled goat anti-human Fc fragment antibody (MP Biomedicals, Solon, Ohio, USA) at a concentration of 0.62 μg/ml was added for 60 min and reacted with tetramethylbenzidine (TMB) substrate. The results were then read on an ELISA reader. Quantisation of the measured infliximab concentration was done by calibration to standard curve in which exogenous infliximab (Schering Plough, New Jersey, USA) was added at concentrations between 10 and 300 ng/ml.
Measurement of ATI
In order to measure ATI we have exploited the fact that the infliximab molecule is composed of two κ chains and have developed an ELISA assay using a commercially available anti-human λ chain conjugated antibody as the detector antibody. Briefly, infliximab (0.1 mg/ml) was added to pre-plated TNFα (500 ng/ml) in 100 μl wells of ELISA plates (Nunc, Roskilde, Denmark). After drying, 100 μl of serum was added and incubated for 90 min at room temperature. Plates were then washed and goat anti-human λ chain HRP-labelled antibody (Sertec, Oxford, UK) was added at a concentration of 33 ng/ml for 60 min. The results were read by an ELISA reader EL-800 (Biotek Instruments, Winooski, USA) and expressed as μg/ml-equivalent (μg/ml-e) after normalisation versus results obtained using additions of graded concentrations between 10 and 300 ng/ml of goat anti-human F(ab′)2 fragment antibody (MP Biomedicals). For testing cross-reactivity of infliximab exposed and unexposed serums towards adalimumab (Abbott, USA), a similar ELISA as above was performed using 0.1 mg/ml of adalimumab.
Determination of antibodies against fragments of infliximab
Whole infliximab was digested by pepsin (F(ab′)2 fragment) or papain (F(ab′) and Fc fragments). Both digestions, as well as the separation of F(ab′) and Fc fragments, were performed using the respective commercially available kits (kits #44885 and #44888; Pierce, Rockford, Illinois, USA) according to the manufacturer's instructions. The resulting proteins were then used as antigens in the ELISA assays as described above, except for the Fc fragment which does not bind TNFα and was therefore plated directly without pre-plated TNFα. In preliminary experiments using serial dilutions of Fc, F(ab′)2 and F(ab′) fragments, the concentrations yielding optimal OD characteristics on standard curves were 4 μg/ml, 12.5 μg/ml and 60 μg/ml, respectively. These concentrations were therefore used in subsequent ELISA assays of tested sera.
Labelled soluble TNF binding to plate-bound infliximab and to its fragments
TNFα was biotinylated with EZ-link NHS-biotin kit (Pierce) according to the manufacturer's instructions. Infliximab (0.1 mg/ml), F(ab′)2 (12.5 μg/ml) or F(ab′) (60 μg/ml) were added to pre-plated TNFα (750 ng/ml) in ELISA plates (Nunc), and incubated for 60 min at room temperature. After washing, graded concentrations of biotinylated TNFα at the range 1000 ng/ml–10 ng/ml were added and incubated for 60 min. Following washing, 50 ng/ml of streptavidin–HRP (Chemicon, Rosemont, Illinois, USA) was added and incubated for 30 min. TMB substrate was then added at100 μl/well, and the reactions were stopped with 2M H2SO4. The results were read by an ELISA reader EL-800 (Biotek). Similar experiments were performed after addition of ATI+ and ATI− sera at the designated concentrations.
Western blot analysis
Infliximab Fc fraction, 60 μg/lane or F(ab′)2 30 μg/lane were size-separated using a standard 10% SDS polyacrylamide gel. After electrophoresis, proteins were transferred onto nitrocellulose filters (Schleicher and Schuell, Keene, New Hampshire, USA) using a transblot apparatus in a buffer containing 192 mM glycine, 25 mM Tris, 0.025% SDS and 20% methanol. The filter was stained with 0.1% ponceau (Sigma) to assure equal loading and transfer. The filter was blocked by 5% non-fat milk in PBS containing 0.1% Tween 20 (TBS-T) for 1 h at room temperature and then incubated for 2 h at room temperature with tested serum diluted 1:100 in 5% non-fat milk. The filter was washed three times with TBS-T and then incubated with a goat anti-human Fc (when F(ab′)2 antibody tested) or goat anti-human F(ab′)2 antibody (when Fc antibody was tested) at a dilution of 1:20000 at room temperature. Both antibodies were conjugated with HRP (Jackson Immunoresearch Lab, Pennsylvania, USA). The filter was washed three times in TBS-T and developed using the ECL Western blot detection system kit (Pierce) according to the manufacturer's recommended protocol.
Results
Sixty-two infliximab-exposed IBD patients (with 109 serum samples available) and 64 infliximab-naïve controls were tested. Controls consisted of 14 IBD patients, and 50 patients with unrelated disorders or healthy volunteers. The mean levels of infliximab and ATI measured in the control unexposed population were 0.4±0.2 and 0.3±0.45 μg/ml, respectively, and results above the mean+3SD were designated positive. Using these cut-off values, a total of 29 of the 62 (47%) infliximab-exposed patients tested positive for the presence of ATI (figure 1).
Levels of infliximab in serum for infliximab-naive and exposed patients, with breakdown into antibodies to infliximab (ATI) positive and ATI negative infliximab-exposed patients. Each triangle denotes a single serum sample measurement.
Table 1 shows the pertinent clinical characteristics of the infliximab-exposed patients, with breakdown into ATI positive and negative groups. We specifically included in the analysis factors that were previously shown to affect the rate of ATI formation, such as the use of concomitant immunosuppression, pre-infusion intravenous corticosteroids or episodic treatment history.1 4 10 11
Clinical characteristics of the study's infliximab-exposed cohort, stratified into patient groups with or without ATI
Of note, all of the infliximab-exposed patients were naïve to adalimumab at the time of serum sampling (some switched to adalimumab later on). However, to further validate the specificity of our developed ELISA assay for ATI measurement, a sample group of ATI+ serums (n=16) was chosen and tested for the presence of antibodies against adalimumab (ADA); all were found negative. This observation corroborates previous results5 reporting the lack of cross-reactivity of ATI with adalimumab and lends further support to the specificity of the present assay.
We next aimed to investigate the immunogenic component of the infliximab molecule. Following enzymatic digestion and separation of infliximab to its Fc, F(ab′) and F(ab′)2 fragments, these fragments were employed as antigens in ELISA assays for measurement of antibodies in serum. As figure 2A shows, a significant portion of patients treated by infliximab had detectable anti-F(ab′)2 and anti-F(ab′) antibodies, yielding mean antibody levels that were significantly higher than the mean reading for the infliximab-naïve patients (6.2±7.1 vs 0.9±0.8 and 3.6±3.7 vs 1.5±0.9 for anti- F(ab′)2 and anti-F(ab′) in infliximab-exposed versus infliximab naïve, respectively; p<0.001 for both comparisons). In contrast, no difference in the mean anti-Fc antibody level was evident between infliximab-naïve and -exposed groups, indicating that no specific immune response towards the Fc fragment could be attributed to infliximab exposure (figure 2A). To validate the results pertaining to the antigenic fragment, a western blotting was performed using positive ATI serums from 11 patients and 10 serums from infliximab-naïve patients. As shown in a representative strip of eight tested samples (figure 2B), anti-F(ab′)2 antibodies—but not anti-Fc antibodies—were present in infliximab-exposed patients, whereas neither antibody was detected in naïve controls. Interestingly, the presence of anti-F(ab′)2 and anti-F(ab′) antibodies was not restricted only to ATI+ sera, as can be seen in the analysis of all 109 available samples from the 62 infliximab-exposed patients (table 2).
(A) Dot plots of antibody levels (in μg/ml-equivalent) to the respective antigens in serums of infliximab-exposed compared to infliximab-naïve patients. Each triangle represents the result obtained for an individual patient. IFX, infliximab; ATI, antibodies to infliximab; mcg/ml-e, μg/ml-equivalent. (B) Western blot analysis of serum reactions to F(ab′)2 fragment of infliximab (upper panel) versus reactions to infliximab's Fc portion (lower panel), and to control lanes in which anti-F(ab′)2 and anti-Fc were employed (denoted as F(ab′)2 and Fc lanes, respectively). IFx, infliximab-exposed patient; NC, naïve controls unexposed to infliximab.
The percentage of detectable anti-infliximab fragments antibodies and detectable infliximab in sera with or without measurable ATI
Conceptually, the binding of infliximab and its F(ab′) and F(ab′)2 fragments to the plastic via pre-plated TNF could possibly block the paratopes from binding by anti-idiotypic antibodies. However, subsequent experiments showed that plate-bound infliximab and F(ab′)2 were still able to bind soluble biotinylated TNF. These findings suggest that at least some of their paratopic sites are still exposed to anti-idiotypic antibodies in serum (figure 3A). This was further supported by competition assays showing that ATI+ sera significantly decreased the remnant TNF binding capacity of plate-bound infliximab, whereas ATI− sera did not (figure 3B). In contrast, the F(ab′) fragment was not able to bind soluble exogenous TNF (figure 3A), possibly suggesting that the measured anti-F(ab′) reactions may comprise predominantly or entirely of antibodies directed against antigenic determinants outside the paratopic loci.
(A) Binding of biotinylated soluble tumour necrosis factor (TNF) by infliximab, F(ab′)2 and F(ab′) after their coupling to ELISA plates via plate-bound TNFα. Biotinylated TNFα was added at the designated concentrations, then incubated with streptavidin–HRP followed by TMB substrate. Results are expressed as optical density (OD). (B) Binding of biotinylated soluble TNFα by infliximab measured as above after addition of either ATI+ sera (continuous lines, n=4) or ATI− sera (dashed lines, n=2). Results were expressed as the measured TNFα binding for each measurement divided by the measurement obtained in the absence of any additional serum and multiplied by 100 (binding capacity). ATI, antibodies to infliximab.
Finally, the correlation between clinical response to infliximab and the ATI level was compared to the correlation of clinical response with anti-F(ab′) or anti-F(ab′)2 level. For this purpose, we examined the levels of antibodies and infliximab trough levels measured in patients who have completely lost response to infliximab vis-à-vis patients who maintained their response to 5 mg/kg/8 week scheduled infliximab treatment (figure 4). We also assessed separately a group of patients who have regained their clinical response following dose intensification after prior loss of response. The results showed that 18 of 24 (78%) responding patients were negative for ATI compared with only 2/12 (17%) of patients with complete loss of response (p=0.001, Fisher exact test). Among the few responding patients with measurable ATI, the titre of ATI was notably low in all but one (<4 μg/ml-e) whereas high ATI titre (>5 μg/ml-e, figure 4) was commonly found among patients who have lost clinical response. Patients who regained response to infliximab consisted of a heterogeneous group comprised of patients with infliximab+/ATI− as well as infliximab−/ATI+ profiles. Finally, a multivariate analysis was performed to investigate the independence of association of ATI+ status with loss of response. This analysis showed that ATI-positivity was the only parameter that independently increased the odds for loss of response (table 3), although the relatively small sample size resulted in a wide CI.
Dot plots of antibodies to infliximab (ATI) levels and infliximab trough level for the three patient groups (responding to maintenance 5 mg/kg/8 week, regaining response to intensified treatment or completely losing response to infliximab). Each dot represents the result obtained from an individual patient. The dashed lines denote the assay's lower limit of detection for the respective parameter. The two arrows denote two specific patients whose case is described briefly in the text. ATI, antibodies to infliximab; mcg/ml-e, μg/ml-equivalent.
Multivariate analysis showing the OR for loss of response for the various parameters tested
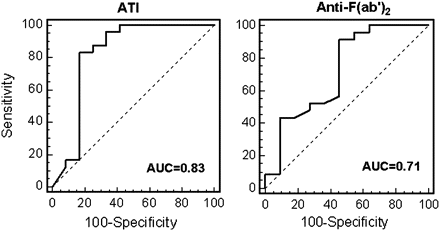
A similar analysis was undertaken to investigate the clinical relevance of levels of anti-F(ab′)2 antibodies (figure 5). Most patients with loss of response were found positive for the presence of anti-F(ab′)2 antibodies, similar to ATI results. However, in contrast with ATI, a significant fraction of responding patients was also found positive for anti-F(ab′)2. The rate of anti-F(ab′)2 positivity in this group was not different than the rate among the patients with complete loss of response (13/24 vs 9/12, respectively, p=0.3), thereby limiting the clinical usefulness of anti-F(ab′)2 determination. When plotting ROC curves for the two variables, this has manifested as an AUC of 0.82 for ATI, while AUC for anti-F(ab′)2 was lower at 0.71 (figure 6). Presence or absence of anti- F(ab′) antibodies was also not significantly different between the two groups (data not shown). Collectively, these data suggest that ATI levels are better correlated with clinical response (or its loss) compared to anti-F(ab′)2 levels.
Dot plots of anti-F(ab′)2 levels and infliximab trough levels for the three patient groups (responding to maintenance 5 mg/kg/8 week, regained response to intensified treatment or completely losing response to infliximab). Each dot represents the result obtained from an individual patient. The dashed lines denote the assay's lower limit of detection for the respective parameter. mcg/ml-e, μg/ml-equivalent.
ROC curves for test accuracy for loss of response for antibodies to infliximab (left figure) and anti-F(ab′)2 (right figure). AUC, area under the curve; ATI, antibodies to infliximab.
Discussion
This study aimed to identify the immunogenic fragment of the infliximab molecule, and to elucidate the potential usefulness of determining levels of antibodies to this fragment as a clinical tool for the evaluation of infliximab-treated patients with secondary loss of response.
Infliximab is a chimeric IgG1 construct composed of human Fc and murine-derived F(ab′) fragments. While the murine xenogenic portion of the F(ab′) would be the more likely fragment to elicit immunogenicity in man, the human Fc may also be able to invoke the formation of anti-allotypic antibodies, at least on a conceptual level.12 Notwithstanding, only a single prior study, in patients with rheumatoid arthritis, assessed the immunogenic part of the infliximab molecule and suggested it to be the F(ab′) fragment,9 and no such study has been performed in patients with Crohn's disease. The present findings corroborate these results by showing the lack of detectable antibodies to infliximab Fc fragment and the presence of anti-F(ab′)2 and anti F(ab′) antibodies among infliximab-exposed patients by two separate techniques (ELISA and western blotting). Although both are solid-phase assays, the dual technique employed lends further validity to the observed results, which are also in line with a previous study that failed to show linkage between FcG1m allotypic divergence and ATI positivity.13 Interestingly, there was a non-negligible portion of patients who had only the latter antibodies in the face of undetectable ATI. This could result from unmasking of epitopes by enzymatic digestion of the intact molecule, which in turn may be recognised by serum antibodies which have been otherwise undetected by an assay employing the whole molecule as the antigen.
There have been conflicting reports on the correlation between ATI level and response to infliximab. Baert et al, using a double-antigen ELISA technique, showed that high levels of ATI correlated with low trough levels of infliximab and secondary loss of response as well as with an increased rate of infusion reactions.3 Similar results were shown by other investigators who used a fluid phase RIA-based assay for functional TNF-binding capacity,5 an RIA-based measurement of pepsin-treated infliximab binding to protein A-fixed serum immunoglobulines,6 or a TNFα binding neutralisation assay.14 In contrast, no correlation of ATI with clinical efficacy of infliximab during 102 week duration of administration was found by the ATTRACT investigators in patients with rheumatoid arthritis.7 Similarly, ATI were detected in only a minority of infliximab-treated IBD patients after withdrawal of concomitant thiopurines, including in the relapsing patients, or in those with low trough levels of the drug.15
These conflicting results attest to the gaps in our understanding of the impact of ATI, and may also stem from dissimilar populations studied or from disparate techniques used. Conceptually, suboptimal test performance could be also improved by an alternative detection assay employing a more purified antigenic component of the infliximab moiety. This approach has proved useful in increasing test specificity and/or sensitivity in other assays of antigenic driven immune disorders; for instance, in the detection of antibodies to pyruvate dehydrogenase sub-units versus anti-mitochondrial antibodies for the diagnosis of primary biliary cirrhosis.16 Notwithstanding, despite the F(ab′)2 being the immunogenic locus of infliximab, our results indicate for the first time that measurement of antibodies against the F(ab′)2 fragment offers no diagnostic advantage over ATI determination, and may in fact be inferior to it.
While the F(ab′)2 antibodies failed to show clinical usefulness, our study reaffirmed that low infliximab trough levels and high ATI levels are strongly correlated with the loss of response to infliximab in IBD patients. It is still unclear why this is replicated in some studies but not all. The different populations studied, the diverse techniques used, the dissimilar time-points of measurement (ie, trough versus 4 weeks post-infusion) and the disparate units of measurements reported, may all be responsible for these discrepant results. Comparative studies assessing side-by-side the accuracy and clinical relevance of the different assays are pertinent before firm conclusions can be drawn on the most useful and applicable assay for clinical use.
Algorithms translating infliximab and ATI levels into management decisions in patients with loss of response have also been proposed.12 17 Our study was not designed to specifically address therapeutic choices and should therefore be interpreted cautiously in this context. However, the fact that the ATI test accuracy for clinical response was seemingly sub-optimal with AUC=0.81 may attest to the presence of other causes for loss of response other than immunogenicity in some cases. Indeed, one patient with ileal Crohn's disease was switched to adalimumab following loss of response to infliximab despite high infliximab trough levels and absent ATI (marked by hollow arrow in left graph, figure 3). The patient had normal C-reactive protein (CRP), two miniature erosions on ileoscopy and no stricture on MR-enterography. She has subsequently failed to respond to adalimumab, but partially responded to IBS-directed medications. Mirroring this from an opposite angle, one patient with ileocolonic Crohn's disease treated by intensified infliximab/4 weeks has had 2 years of regained clinical remission despite undetectable infliximab trough levels and high ATI titre (patient is denoted by a solid arrow in right graph, figure 3). This assay result prompted re-evaluation which revealed normal CRP levels and totally normal ileocolonoscopy. Infliximab was subsequently stopped and the patient has since remained in remission for over 9 months and until the end of follow-up. Albeit limited, this patient's course is novel for suggesting that measurement of infliximab and ATI level may prove helpful not only for the management of patients who lose response, but perhaps also for patients in seemingly maintained response to prolonged administration of infliximab, who may in fact be enjoying a 'self-maintained' remission and are no longer benefiting from the drug. This reasoning may account for the otherwise puzzling observation of a recent clinical study, in which the ability to stop infliximab therapy and maintain clinical remission was predicted by low infliximab levels.18 In practical terms, clinicians should measure infliximab trough levels in patients with secondary loss of response; high levels should guide a search for other causes for the patient's symptoms, or a shift in the predominant inflammatory pathway. Low infliximab levels should prompt ATI measurement. ATI negativity would suggest drug failure due to non-immune drug clearance and/or increased TNF tissue burden and justify dose-intensification, although it is also possible that some cases of ATI negativity are due to rapid immune-complexes clearance. In contrast, ATI positivity would indicate immune loss-of-response for which dose escalation with adjunct immunomodulators or a switch to another anti-TNF should probably be considered. It remains to be determined by future studies whether the presence of measurable ATI with detectable infliximab in serum (as occurred in 33% of our ATI+ samples) is a forerunner for immune loss-of-response that merits early preemptive interventions.
Similar to other studies, we also found significantly higher hypersensitivity reactions among ATI+ patients compared to ATI negative ones (table 1). There was also a numeric trend for increased risk of ATI in patients under episodic therapy or those not receiving concomitant immunesuppressors. However, this tendency did not reach statistical significance, most likely due to the small numbers of patients treated episodically in our cohort, as it was shown that it is this episodic group that enjoys the protective effect of concomitant immunomodulators the most.11
A notable limitation of our study is that the clinical outcome of patients was not evaluated in a standardised manner using accepted clinical scores. Nevertheless, this limitation is shared by most studies on this topic and may not hamper the utility of the results as reflecting common clinical practice. A technical limitation of the ATI and anti-F(ab′)2 assay should also be noted; it cannot detect purely κ containing antibodies and may also be amenable to false positive results from low affinity antibodies and from pepsin agglutinators.12
In conclusion, the immunogenic component of the infliximab molecule lies within the F(ab′)2 fragment. However, ATI, that is antibodies against the intact molecule, are more strongly associated with maintained response to infliximab or lack of response. Thus, management decisions are more likely to benefit from infliximab trough level in conjunction with ATI determination rather than from anti-F(ab′)2 levels.
References
Footnotes
Funding The study was supported in part by a ‘Talpiot’ unrestricted research grant from the Sheba Medical Center (to SBH).
Competing interests SBH and YC have received consultancy fees from Abbott Laboratories and Schering-Plough.
Ethics approval This study was conducted with the approval of the Sheba Medical Center.
Provenance and peer review Not commissioned; externally peer reviewed.