Endothelial Aging Associated with Oxidative Stress Can Be Modulated by a Healthy Mediterranean Diet
Abstract
:1. Introduction
2. Vascular Aging and Oxidative Stress
3. Vascular Aging and Inflammation
4. Vascular Aging and Alteration of the NO Pathway
4.1. Deficiency in NOS Substrates and Cofactors
4.2. Presence of Endogenous eNOS Inhibitors
4.3. Lower Expression and/or Activity of eNOS
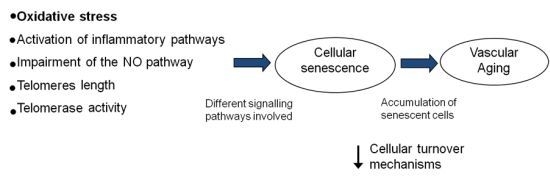
5. Vascular Aging and Cellular Senescence
5.1. Number and Function of Endothelial Progenitor Cells (EPCs)
5.2. Impaired Cell Replication and Telomere Shortening in Aged Arteries
5.3. Microparticles
6. Mediterranean Diet: Does It Prevent Endothelial Aging?
7. Conclusions and Future Expectations
Acknowledgments
Conflict of Interest
References
- Mirea, O.; Donoiu, I.; Plesea, I.E. Arterial aging: A brief review. Rom. J. Morphol. Embryol 2012, 53, 473–477. [Google Scholar]
- Sohal, R.S.; Mockett, R.J.; Orr, W.C. Mechanisms of aging: An appraisal of the oxidative stress hypothesis. Free Radic Biol. Med 2002, 33, 575–586. [Google Scholar]
- Ferrari, A.U.; Radaelli, A.; Centola, M. Invited review: Aging and the cardiovascular system. J. Appl. Physiol 2003, 95, 2591–2597. [Google Scholar]
- Panza, J.A.; Quyyumi, A.A.; Brush, J.E., Jr; Epstein, S.E. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N. Engl. J. Med 1990, 323, 22–27. [Google Scholar]
- Yildiz, O. Vascular smooth muscle and endothelial functions in aging. Ann. N. Y. Acad. Sci 2007, 1100, 353–360. [Google Scholar]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med 1993, 329, 2002–2012. [Google Scholar]
- Barodka, V.M.; Joshi, B.L.; Berkowitz, D.E.; Hogue, C.W., Jr; Nyhan, D. Review article: Implications of vascular aging. Anesth. Analg 2011, 112, 1048–1060. [Google Scholar]
- Rodriguez-Manas, L.; El-Assar, M.; Vallejo, S.; Lopez-Doriga, P.; Solis, J.; Petidier, R.; Montes, M.; Nevado, J.; Castro, M.; Gomez-Guerrero, C.; et al. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell 2009, 8, 226–238. [Google Scholar]
- Irani, K. Oxidant signaling in vascular cell growth, death, and survival : A review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ. Res 2000, 87, 179–183. [Google Scholar]
- Seals, D.R.; Jablonski, K.L.; Donato, A.J. Aging and vascular endothelial function in humans. Clin. Sci 2011, 120, 357–375. [Google Scholar]
- Lloyd-Jones, D.; Adams, R.J.; Brown, T.M.; Carnethon, M.; Dai, S.; de Simone, G.; Ferguson, T.B.; Ford, E.; Furie, K.; Gillespie, C.; et al. Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation 2010, 121, e46–e215. [Google Scholar]
- Ungvari, Z.; Kaley, G.; de Cabo, R.; Sonntag, W.E.; Csiszar, A. Mechanisms of vascular aging: New perspectives. J. Gerontol. A 2010, 65, 1028–1041. [Google Scholar]
- Van der Loo, B.; Labugger, R.; Skepper, J.N.; Bachschmid, M.; Kilo, J.; Powell, J.M.; Palacios-Callender, M.; Erusalimsky, J.D.; Quaschning, T.; Malinski, T.; et al. Enhanced peroxynitrite formation is associated with vascular aging. J. Exp. Med 2000, 192, 1731–1744. [Google Scholar]
- Donato, A.J.; Eskurza, I.; Silver, A.E.; Levy, A.S.; Pierce, G.L.; Gates, P.E.; Seals, D.R. Direct evidence of endothelial oxidative stress with aging in humans: Relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ. Res 2007, 100, 1659–1666. [Google Scholar]
- Toda, N. Age-related changes in endothelial function and blood flow regulation. Pharmacol. Ther 2012, 133, 159–176. [Google Scholar]
- Virdis, A.; Ghiadoni, L.; Giannarelli, C.; Taddei, S. Endothelial dysfunction and vascular disease in later life. Maturitas 2010, 67, 20–24. [Google Scholar]
- D’Alessio, P. Aging and the endothelium. Exp. Gerontol 2004, 39, 165–171. [Google Scholar]
- Ferrucci, L.; Corsi, A.; Lauretani, F.; Bandinelli, S.; Bartali, B.; Taub, D.D.; Guralnik, J.M.; Longo, D.L. The origins of age-related proinflammatory state. Blood 2005, 105, 2294–2299. [Google Scholar]
- Ungvari, Z.; Csiszar, A.; Kaminski, P.M.; Wolin, M.S.; Koller, A. Chronic high pressure-induced arterial oxidative stress: Involvement of protein kinase C-dependent NAD(P)H oxidase and local renin-angiotensin system. Am. J. Pathol 2004, 165, 219–226. [Google Scholar]
- Minamino, T.; Komuro, I. Role of telomeres in vascular senescence. Front. Biosci 2008, 13, 2971–2979. [Google Scholar]
- Ogami, M.; Ikura, Y.; Ohsawa, M.; Matsuo, T.; Kayo, S.; Yoshimi, N.; Hai, E.; Shirai, N.; Ehara, S.; Komatsu, R.; et al. Telomere shortening in human coronary artery diseases. Arterioscler. Thromb. Vasc. Biol 2004, 24, 546–550. [Google Scholar]
- Keymel, S.; Kalka, C.; Rassaf, T.; Yeghiazarians, Y.; Kelm, M.; Heiss, C. Impaired endothelial progenitor cell function predicts age-dependent carotid intimal thickening. Basic Res. Cardiol 2008, 103, 582–586. [Google Scholar]
- Kirton, J.P.; Xu, Q. Endothelial precursors in vascular repair. Microvasc. Res 2010, 79, 193–199. [Google Scholar]
- Yubero-Serrano, E.M.; Delgado-Casado, N.; Delgado-Lista, J.; Perez-Martinez, P.; Tasset-Cuevas, I.; Santos-Gonzalez, M.; Caballero, J.; Garcia-Rios, A.; Marin, C.; Gutierrez-Mariscal, F.M.; et al. Postprandial antioxidant effect of the Mediterranean diet supplemented with coenzyme Q10 in elderly men and women. Age 2011, 33, 579–590. [Google Scholar]
- Yubero-Serrano, E.M.; Garcia-Rios, A.; Delgado-Lista, J.; Delgado-Casado, N.; Perez-Martinez, P.; Rodriguez-Cantalejo, F.; Fuentes, F.; Cruz-Teno, C.; Tunez, I.; Tasset-Cuevas, I.; et al. Postprandial effects of the Mediterranean diet on oxidant and antioxidant status in elderly men and women. J. Am. Geriatr. Soc 2011, 59, 938–940. [Google Scholar]
- McCarty, M.F. A low-fat, whole-food vegan diet, as well as other strategies that down-regulate IGF-I activity, may slow the human aging process. Med. Hypotheses 2003, 60, 784–792. [Google Scholar]
- Csiszar, A.; Labinskyy, N.; Pinto, J.T.; Ballabh, P.; Zhang, H.; Losonczy, G.; Pearson, K.; de Cabo, R.; Pacher, P.; Zhang, C.; et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am. J. Physiol. Heart Circ. Physiol 2009, 297, H13–H20. [Google Scholar]
- Tang, Y.; Xu, J.; Qu, W.; Peng, X.; Xin, P.; Yang, X.; Ying, C.; Sun, X.; Hao, L. Resveratrol reduces vascular cell senescence through attenuation of oxidative stress by SIRT1/NADPH oxidase-dependent mechanisms. J. Nutr. Biochem 2012, 23, 1410–1416. [Google Scholar]
- Landberg, R.; Naidoo, N.; van Dam, R.M. Diet and endothelial function: From individual components to dietary patterns. Curr. Opin. Lipidol 2012, 23, 147–155. [Google Scholar]
- Kiecolt-Glaser, J.K.; Belury, M.A.; Andridge, R.; Malarkey, W.B.; Hwang, B.S.; Glaser, R. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: A randomized controlled trial. Brain Behav. Immun 2012, 26, 988–995. [Google Scholar]
- Kiecolt-Glaser, J.K.; Epel, E.S.; Belury, M.A.; Andridge, R.; Lin, J.; Glaser, R.; Malarkey, W.B.; Hwang, B.S.; Blackburn, E. Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: A randomized controlled trial. Brain Behav. Immun 2013, 28, 16–24. [Google Scholar]
- Weiss, E.P.; Fontana, L. Caloric restriction: powerful protection for the aging heart and vasculature. Am. J. Physiol. Heart Circ. Physiol 2011, 301, H1205–H1219. [Google Scholar]
- Scoditti, E.; Calabriso, N.; Massaro, M.; Pellegrino, M.; Storelli, C.; Martines, G.; de Caterina, R.; Carluccio, M.A. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: A potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch. Biochem. Biophys 2012, 527, 81–89. [Google Scholar]
- Mirabello, L.; Huang, W.Y.; Wong, J.Y.; Chatterjee, N.; Reding, D.; Crawford, E.D.; de Vivo, I.; Hayes, R.B.; Savage, S.A. The association between leukocyte telomere length and cigarette smoking, dietary and physical variables, and risk of prostate cancer. Aging Cell 2009, 8, 405–413. [Google Scholar]
- Marin, C.; Delgado-Lista, J.; Ramirez, R.; Carracedo, J.; Caballero, J.; Perez-Martinez, P.; Gutierrez-Mariscal, F.M.; Garcia-Rios, A.; Delgado-Casado, N.; Cruz-Teno, C.; et al. Mediterranean diet reduces senescence-associated stress in endothelial cells. Age 2012, 34, 1309–1316. [Google Scholar]
- Marin, C.; Ramirez, R.; Delgado-Lista, J.; Yubero-Serrano, E.M.; Perez-Martinez, P.; Carracedo, J.; Garcia-Rios, A.; Rodriguez, F.; Gutierrez-Mariscal, F.M.; Gomez, P.; et al. Mediterranean diet reduces endothelial damage and improves the regenerative capacity of endothelium. Am. J. Clin. Nutr 2011, 93, 267–274. [Google Scholar]
- Fernandez, J.M.; Rosado-Alvarez, D.; da Silva Grigoletto, M.E.; Rangel-Zuniga, O.A.; Landaeta-Diaz, L.L.; Caballero-Villarraso, J.; Lopez-Miranda, J.; Perez-Jimenez, F.; Fuentes-Jimenez, F. Moderate-to-high-intensity training and a hypocaloric Mediterranean diet enhance endothelial progenitor cells and fitness in subjects with the metabolic syndrome. Clin. Sci 2012, 123, 361–373. [Google Scholar]
- Perez-Martinez, P.; Garcia-Quintana, J.M.; Yubero-Serrano, E.M.; Tasset-Cuevas, I.; Tunez, I.; Garcia-Rios, A.; Delgado-Lista, J.; Marin, C.; Perez-Jimenez, F.; Roche, H.M.; et al. Postprandial oxidative stress is modified by dietary fat: Evidence from a human intervention study. Clin. Sci 2010, 119, 251–261. [Google Scholar]
- Cruz-Teno, C.; Perez-Martinez, P.; Delgado-Lista, J.; Yubero-Serrano, E.M.; Garcia-Rios, A.; Marin, C.; Gomez, P.; Jimenez-Gomez, Y.; Camargo, A.; Rodriguez-Cantalejo, F.; et al. Dietary fat modifies the postprandial inflammatory state in subjects with metabolic syndrome: the LIPGENE study. Mol. Nutr. Food Res 2012, 56, 854–865. [Google Scholar]
- Cassidy, A.; de Vivo, I.; Liu, Y.; Han, J.; Prescott, J.; Hunter, D.J.; Rimm, E.B. Associations between diet, lifestyle factors, and telomere length in women. Am. J. Clin. Nutr 2011, 91, 1273–1280. [Google Scholar]
- Farzaneh-Far, R.; Lin, J.; Epel, E.S.; Harris, W.S.; Blackburn, E.H.; Whooley, M.A. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA 2010, 303, 250–257. [Google Scholar]
- Jung, K.J.; Lee, E.K.; Kim, J.Y.; Zou, Y.; Sung, B.; Heo, H.S.; Kim, M.K.; Lee, J.; Kim, N.D.; Yu, B.P.; et al. Effect of short term calorie restriction on pro-inflammatory NF-κB and AP-1 in aged rat kidney. Inflamm. Res 2009, 58, 143–150. [Google Scholar]
- Zou, Y.; Yoon, S.; Jung, K.J.; Kim, C.H.; Son, T.G.; Kim, M.S.; Kim, Y.J.; Lee, J.; Yu, B.P.; Chung, H.Y. Upregulation of aortic adhesion molecules during aging. J. Gerontol. A 2006, 61, 232–244. [Google Scholar]
- Ungvari, Z.; Orosz, Z.; Labinskyy, N.; Rivera, A.; Xiangmin, Z.; Smith, K.; Csiszar, A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am. J. Physiol. Heart Circ. Physiol 2007, 293, H37–H47. [Google Scholar]
- Csiszar, A.; Smith, K.; Labinskyy, N.; Orosz, Z.; Rivera, A.; Ungvari, Z. Resveratrol attenuates TNF-alpha-induced activation of coronary arterial endothelial cells: Role of NF-kappaB inhibition. Am. J. Physiol. Heart Circ. Physiol 2006, 291, H1694–H1699. [Google Scholar]
- Goraca, A. Assessment of total antioxidant capacity in human plasma. Folia Med 2004, 46, 16–21. [Google Scholar]
- Hossain, M.; Qadri, S.M.; Liu, L. Inhibition of nitric oxide synthesis enhances leukocyte rolling and adhesion in human microvasculature. J. Inflamm. (Lond.) 2012, 9, 28. [Google Scholar]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev 2007, 87, 315–424. [Google Scholar]
- Mecocci, P.; Parnetti, L.; Romano, G.; Scarelli, A.; Chionne, F.; Cecchetti, R.; Polidori, M.C.; Palumbo, B.; Cherubini, A.; Senin, U. Serum anti-GFAP and anti-S100 autoantibodies in brain aging, Alzheimer’s disease and vascular dementia. J. Neuroimmunol 1995, 57, 165–170. [Google Scholar]
- Pang, L.J.; Shao, J.Y.; Liang, X.M.; Xia, Y.F.; Zeng, Y.X. Mitochondrial DNA somatic mutations are frequent in nasopharyngeal carcinoma. Cancer Biol. Ther 2008, 7, 198–207. [Google Scholar]
- Hamilton, C.A.; Brosnan, M.J.; McIntyre, M.; Graham, D.; Dominiczak, A.F. Superoxide excess in hypertension and aging: A common cause of endothelial dysfunction. Hypertension 2001, 37, 529–534. [Google Scholar]
- Yang, Y.M.; Huang, A.; Kaley, G.; Sun, D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am. J. Physiol. Heart Circ. Physiol 2009, 297, H1829–H1836. [Google Scholar]
- Ungvari, Z.; Buffenstein, R.; Austad, S.N.; Podlutsky, A.; Kaley, G.; Csiszar, A. Oxidative stress in vascular senescence: lessons from successfully aging species. Front. Biosci 2008, 13, 5056–5070. [Google Scholar]
- Li, M.; Fukagawa, N.K. Age-related changes in redox signaling and VSMC function. Antioxid. Redox Signal 2010, 12, 641–655. [Google Scholar]
- Malinin, N.L.; West, X.Z.; Byzova, T.V. Oxidation as “the stress of life”. Aging 2011, 3, 906–910. [Google Scholar]
- Csiszar, A.; Wang, M.; Lakatta, E.G.; Ungvari, Z. Inflammation and endothelial dysfunction during aging: Role of NF-kappaB. J. Appl. Physiol 2008, 105, 1333–1341. [Google Scholar]
- Csiszar, A.; Toth, J.; Peti-Peterdi, J.; Ungvari, Z. The aging kidney: role of endothelial oxidative stress and inflammation. Acta Physiol. Hung 2007, 94, 107–115. [Google Scholar]
- Ungvari, Z.; Parrado-Fernandez, C.; Csiszar, A.; de Cabo, R. Mechanisms underlying caloric restriction and lifespan regulation: Implications for vascular aging. Circ. Res 2008, 102, 519–528. [Google Scholar]
- Loscalzo, J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ. Res 2001, 88, 756–762. [Google Scholar]
- Fukai, T. Extracellular SOD and aged blood vessels. Am. J. Physiol. Heart Circ. Physiol 2009, 297, H10–H12. [Google Scholar]
- Spier, S.A.; Delp, M.D.; Meininger, C.J.; Donato, A.J.; Ramsey, M.W.; Muller-Delp, J.M. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J. Physiol 2004, 556, 947–958. [Google Scholar]
- Bode-Boger, S.M.; Muke, J.; Surdacki, A.; Brabant, G.; Boger, R.H.; Frolich, J.C. Oral l-arginine improves endothelial function in healthy individuals older than 70 years. Vasc. Med 2003, 8, 77–81. [Google Scholar]
- Santhanam, L.; Christianson, D.W.; Nyhan, D.; Berkowitz, D.E. Arginase and vascular aging. J. Appl. Physiol 2008, 105, 1632–1642. [Google Scholar]
- Holowatz, L.A.; Thompson, C.S.; Kenney, W.L. l-Arginine supplementation or arginase inhibition augments reflex cutaneous vasodilatation in aged human skin. J. Physiol 2006, 574, 573–581. [Google Scholar]
- Rodriguez-Crespo, I.; Ortiz de Montellano, P.R. Human endothelial nitric oxide synthase: Expression in Escherichia coli, coexpression with calmodulin, and characterization. Arch. Biochem. Biophys 1996, 336, 151–156. [Google Scholar]
- Channon, K.M.; Watkins, H. Coronary artery disease genetics: Bigger is better. Eur. Heart J 2004, 25, 900–901. [Google Scholar]
- Eskurza, I.; Myerburgh, L.A.; Kahn, Z.D.; Seals, D.R. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J. Physiol 2005, 568, 1057–1065. [Google Scholar]
- Juonala, M.; Viikari, J.S.; Alfthan, G.; Marniemi, J.; Kahonen, M.; Taittonen, L.; Laitinen, T.; Raitakari, O.T. Brachial artery flow-mediated dilation and asymmetrical dimethylarginine in the cardiovascular risk in young Finns study. Circulation 2007, 116, 1367–1373. [Google Scholar]
- Schulze, F.; Maas, R.; Freese, R.; Schwedhelm, E.; Silberhorn, E.; Boger, R.H. Determination of a reference value for N(G), N(G)-dimethyl- l-arginine in 500 subjects. Eur. J. Clin. Invest 2005, 35, 622–626. [Google Scholar]
- Bode-Boger, S.M.; Scalera, F.; Martens-Lobenhoffer, J. Asymmetric dimethylarginine (ADMA) accelerates cell senescence. Vasc. Med 2005, 10, S65–S71. [Google Scholar]
- Matz, R.L.; Schott, C.; Stoclet, J.C.; Andriantsitohaina, R. Age-related endothelial dysfunction with respect to nitric oxide, endothelium-derived hyperpolarizing factor and cyclooxygenase products. Physiol. Res 2000, 49, 11–18. [Google Scholar]
- Zhou, X.; Bohlen, H.G.; Unthank, J.L.; Miller, S.J. Abnormal nitric oxide production in aged rat mesenteric arteries is mediated by NAD(P)H oxidase-derived peroxide. Am. J. Physiol. Heart Circ. Physiol 2009, 297, H2227–H2233. [Google Scholar]
- Donato, A.J.; Gano, L.B.; Eskurza, I.; Silver, A.E.; Gates, P.E.; Jablonski, K.; Seals, D.R. Vascular endothelial dysfunction with aging: Endothelin-1 and endothelial nitric oxide synthase. Am. J. Physiol. Heart Circ. Physiol 2009, 297, H425–H432. [Google Scholar]
- Smith, A.R.; Visioli, F.; Frei, B.; Hagen, T.M. Age-related changes in endothelial nitric oxide synthase phosphorylation and nitric oxide dependent vasodilation: Evidence for a novel mechanism involving sphingomyelinase and ceramide-activated phosphatase 2A. Aging Cell 2006, 5, 391–400. [Google Scholar]
- LeBlanc, A.J.; Shipley, R.D.; Kang, L.S.; Muller-Delp, J.M. Age impairs Flk-1 signaling and NO-mediated vasodilation in coronary arterioles. Am. J. Physiol. Heart Circ. Physiol 2008, 295, H2280–H2288. [Google Scholar]
- Fujiyama, S.; Amano, K.; Uehira, K.; Yoshida, M.; Nishiwaki, Y.; Nozawa, Y.; Jin, D.; Takai, S.; Miyazaki, M.; Egashira, K.; et al. Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ. Res 2003, 93, 980–989. [Google Scholar]
- Ahrens, I.; Domeij, H.; Topcic, D.; Haviv, I.; Merivirta, R.M.; Agrotis, A.; Leitner, E.; Jowett, J.B.; Bode, C.; Lappas, M.; et al. Successful in vitro expansion and differentiation of cord blood derived CD34+ cells into early endothelial progenitor cells reveals highly differential gene expression. PLoS One 2011, 6, e23210. [Google Scholar]
- Thum, T.; Hoeber, S.; Froese, S.; Klink, I.; Stichtenoth, D.O.; Galuppo, P.; Jakob, M.; Tsikas, D.; Anker, S.D.; Poole-Wilson, P.A.; et al. Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1. Circ. Res 2007, 100, 434–443. [Google Scholar]
- Scheubel, R.J.; Zorn, H.; Silber, R.E.; Kuss, O.; Morawietz, H.; Holtz, J.; Simm, A. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J. Am. Coll. Cardiol 2003, 42, 2073–2080. [Google Scholar]
- Heiss, C.; Keymel, S.; Niesler, U.; Ziemann, J.; Kelm, M.; Kalka, C. Impaired progenitor cell activity in age-related endothelial dysfunction. J. Am. Coll. Cardiol 2005, 45, 1441–1448. [Google Scholar]
- Hill, J.M.; Zalos, G.; Halcox, J.P.; Schenke, W.H.; Waclawiw, M.A.; Quyyumi, A.A.; Finkel, T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med 2003, 348, 593–600. [Google Scholar]
- He, T.; Joyner, M.J.; Katusic, Z.S. Aging decreases expression and activity of glutathione peroxidase-1 in human endothelial progenitor cells. Microvasc. Res 2009, 78, 447–452. [Google Scholar]
- Bruunsgaard, H.; Skinhoj, P.; Pedersen, A.N.; Schroll, M.; Pedersen, B.K. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin. Exp. Immunol 2000, 121, 255–260. [Google Scholar]
- Bernardini, D.; Ballabio, E.; Mariotti, M.; Maier, J.A. Differential expression of EDF-1 and endothelial nitric oxide synthase by proliferating, quiescent and senescent microvascular endothelial cells. Biochim. Biophys. Acta 2005, 1745, 265–272. [Google Scholar]
- Ma, F.X.; Zhou, B.; Chen, Z.; Ren, Q.; Lu, S.H.; Sawamura, T.; Han, Z.C. Oxidized low density lipoprotein impairs endothelial progenitor cells by regulation of endothelial nitric oxide synthase. J. Lipid Res 2006, 47, 1227–1237. [Google Scholar]
- Minamino, T.; Miyauchi, H.; Yoshida, T.; Tateno, K.; Komuro, I. The role of vascular cell senescence in atherosclerosis: antisenescence as a novel therapeutic strategy for vascular aging. Curr. Vasc. Pharmacol 2004, 2, 141–148. [Google Scholar]
- Blasco, M.A. Telomeres and human disease: Ageing, cancer and beyond. Nat. Rev. Genet 2005, 6, 611–622. [Google Scholar]
- Aviv, A.; Valdes, A.M.; Spector, T.D. Human telomere biology: Pitfalls of moving from the laboratory to epidemiology. Int. J. Epidemiol 2006, 35, 1424–1429. [Google Scholar]
- Damjanovic, A.K.; Yang, Y.; Glaser, R.; Kiecolt-Glaser, J.K.; Nguyen, H.; Laskowski, B.; Zou, Y.; Beversdorf, D.Q.; Weng, N.P. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. J. Immunol 2007, 179, 4249–4254. [Google Scholar]
- Babizhayev, M.A.; Savel’yeva, E.L.; Moskvina, S.N.; Yegorov, Y.E. Telomere length is a biomarker of cumulative oxidative stress, biologic age, and an independent predictor of survival and therapeutic treatment requirement associated with smoking behavior. Am. J. Ther 2010, 18, e209–e26. [Google Scholar]
- Brouilette, S.; Singh, R.K.; Thompson, J.R.; Goodall, A.H.; Samani, N.J. White cell telomere length and risk of premature myocardial infarction. Arterioscler. Thromb. Vasc. Biol 2003, 23, 842–846. [Google Scholar]
- Jaskelioff, M.; Muller, F.L.; Paik, J.H.; Thomas, E.; Jiang, S.; Adams, A.C.; Sahin, E.; Kost-Alimova, M.; Protopopov, A.; Cadinanos, J.; et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 2011, 469, 102–106. [Google Scholar]
- Sahin, E.; Colla, S.; Liesa, M.; Moslehi, J.; Muller, F.L.; Guo, M.; Cooper, M.; Kotton, D.; Fabian, A.J.; Walkey, C.; et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 2011, 470, 359–365. [Google Scholar]
- Olivieri, F.; Recchioni, R.; Marcheselli, F.; Abbatecola, A.M.; Santini, G.; Borghetti, G.; Antonicelli, R.; Procopio, A.D. Cellular senescence in cardiovascular diseases: Potential age-related mechanisms and implications for treatment. Curr. Pharm. Des 2012, 19, 1710–1719. [Google Scholar]
- Boulanger, C.M.; Amabile, N.; Tedgui, A. Circulating microparticles: A potential prognostic marker for atherosclerotic vascular disease. Hypertension 2006, 48, 180–186. [Google Scholar]
- Sabatier, F.; Roux, V.; Anfosso, F.; Camoin, L.; Sampol, J.; Dignat-George, F. Interaction of endothelial microparticles with monocytic cells in vitro induces tissue factor-dependent procoagulant activity. Blood 2002, 99, 3962–3970. [Google Scholar]
- Baron, M.; Boulanger, C.M.; Staels, B.; Tailleux, A. Cell-derived microparticles in atherosclerosis: Biomarkers and targets for pharmacological modulation? J. Cell. Mol. Med 2011, 16, 1365–1376. [Google Scholar]
- Gyorgy, B.; Szabo, T.G.; Pasztoi, M.; Pal, Z.; Misjak, P.; Aradi, B.; Laszlo, V.; Pallinger, E.; Pap, E.; Kittel, A.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci 2011, 68, 2667–2688. [Google Scholar]
- Esposito, K.; Ciotola, M.; Schisano, B.; Gualdiero, R.; Sardelli, L.; Misso, L.; Giannetti, G.; Giugliano, D. Endothelial microparticles correlate with endothelial dysfunction in obese women. J. Clin. Endocrinol. Metab 2006, 91, 3676–3679. [Google Scholar]
- McDonald, A.P.; Meier, T.R.; Hawley, A.E.; Thibert, J.N.; Farris, D.M.; Wrobleski, S.K.; Henke, P.K.; Wakefield, T.W.; Myers, D.D., Jr. Aging is associated with impaired thrombus resolution in a mouse model of stasis induced thrombosis. Thromb. Res 2010, 125, 72–78. [Google Scholar]
- Previtali, E.; Bucciarelli, P.; Passamonti, S.M.; Martinelli, I. Risk factors for venous and arterial thrombosis. Blood Transfus 2011, 9, 120–138. [Google Scholar]
- Brodsky, S.V.; Zhang, F.; Nasjletti, A.; Goligorsky, M.S. Endothelium-derived microparticles impair endothelial function in vitro. Am. J. Physiol. Heart Circ. Physiol 2004, 286, H1910–H1915. [Google Scholar]
- Burger, D.; Montezano, A.C.; Nishigaki, N.; He, Y.; Carter, A.; Touyz, R.M. Endothelial microparticle formation by angiotensin II is mediated via Ang II receptor type I/NADPH oxidase/Rho kinase pathways targeted to lipid rafts. Arterioscler. Thromb. Vasc. Biol 2011, 31, 1898–1907. [Google Scholar]
- Terrisse, A.D.; Puech, N.; Allart, S.; Gourdy, P.; Xuereb, J.M.; Payrastre, B.; Sie, P. Internalization of microparticles by endothelial cells promotes platelet/endothelial cell interaction under flow. J. Thromb. Haemost 2010, 8, 2810–2819. [Google Scholar]
- Yang, C.; Mwaikambo, B.R.; Zhu, T.; Gagnon, C.; Lafleur, J.; Seshadri, S.; Lachapelle, P.; Lavoie, J.C.; Chemtob, S.; Hardy, P. Lymphocytic microparticles inhibit angiogenesis by stimulating oxidative stress and negatively regulating VEGF-induced pathways. Am. J. Physiol. Regul. Integr. Comp. Physiol 2008, 294, R467–R476. [Google Scholar]
- Essayagh, S.; Xuereb, J.M.; Terrisse, A.D.; Tellier-Cirioni, L.; Pipy, B.; Sie, P. Microparticles from apoptotic monocytes induce transient platelet recruitment and tissue factor expression by cultured human vascular endothelial cells via a redox-sensitive mechanism. Thromb. Haemost 2007, 98, 831–837. [Google Scholar]
- Klonizakis, M.; Alkhatib, A.; Middleton, G.; Smith, M.F. Mediterranean diet- and exercise-induced improvement in age-dependent vascular activity. Clin. Sci 2012, 124, 579–587. [Google Scholar]
- Dal-Ros, S.; Bronner, C.; Auger, C.; Schini-Kerth, V.B. Red wine polyphenols improve an established aging-related endothelial dysfunction in the mesenteric artery of middle-aged rats: Role of oxidative stress. Biochem. Biophys. Res. Commun 2012, 419, 381–387. [Google Scholar]
- Zuchi, C.; Ambrosio, G.; Luscher, T.F.; Landmesser, U. Nutraceuticals in cardiovascular prevention: Lessons from studies on endothelial function. Cardiovasc. Ther 2010, 28, 187–201. [Google Scholar]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Arranz, S.; Valderas-Martinez, P.; Casas, R.; Sacanella, E.; Llorach, R.; Lamuela-Raventos, R.M.; Andres-Lacueva, C.; et al. Dealcoholized red wine decreases systolic and diastolic blood pressure and increases plasma nitric oxide: Short communication. Circ. Res 2012, 111, 1065–1068. [Google Scholar]
- Torabian, S.; Haddad, E.; Rajaram, S.; Banta, J.; Sabate, J. Acute effect of nut consumption on plasma total polyphenols, antioxidant capacity and lipid peroxidation. J. Hum. Nutr. Diet 2009, 22, 64–71. [Google Scholar]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bohn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J 2010, 9, 3. [Google Scholar]
- Sun, Y.; Ma, A.; Li, Y.; Han, X.; Wang, Q.; Liang, H. Vitamin E supplementation protects erythrocyte membranes from oxidative stress in healthy Chinese middle-aged and elderly people. Nutr. Res 2012, 32, 328–334. [Google Scholar]
- Thomas, D.R. Vitamins in aging, health, and longevity. Clin. Interv. Aging 2006, 1, 81–91. [Google Scholar]
- Obrenovich, M.E.; Nair, N.G.; Beyaz, A.; Aliev, G.; Reddy, V.P. The role of polyphenolic antioxidants in health, disease, and aging. Rejuvenation Res 2010, 13, 631–643. [Google Scholar]
- Obrenovich, M.E.; Li, Y.; Parvathaneni, K.; Yendluri, B.B.; Palacios, H.H.; Leszek, J.; Aliev, G. Antioxidants in health, disease and aging. CNS Neurol. Disord. Drug Targets 2011, 10, 192–207. [Google Scholar]
- Jennings, B.J.; Ozanne, S.E.; Dorling, M.W.; Hales, C.N. Early growth determines longevity in male rats and may be related to telomere shortening in the kidney. FEBS Lett 1999, 448, 4–8. [Google Scholar]
- Shen, J.; Gammon, M.D.; Terry, M.B.; Wang, Q.; Bradshaw, P.; Teitelbaum, S.L.; Neugut, A.I.; Santella, R.M. Telomere length, oxidative damage, antioxidants and breast cancer risk. Int. J. Cancer 2009, 124, 1637–1643. [Google Scholar]
- Erusalimsky, J.D.; Skene, C. Mechanisms of endothelial senescence. Exp. Physiol 2009, 94, 299–304. [Google Scholar]
- Meydani, A.; Ahmed, T.; Meydani, S.N. Aging, nutritional status, and infection in the developing world. Nutr. Rev 2005, 63, 233–246. [Google Scholar]
- Esposito, K.; di Palo, C.; Maiorino, M.I.; Petrizzo, M.; Bellastella, G.; Siniscalchi, I.; Giugliano, D. Long-term effect of mediterranean-style diet and calorie restriction on biomarkers of longevity and oxidative stress in overweight men. Cardiol. Res. Pract 2011, 2011, 293916. [Google Scholar]
- Everitt, A.V.; Hilmer, S.N.; Brand-Miller, J.C.; Jamieson, H.A.; Truswell, A.S.; Sharma, A.P.; Mason, R.S.; Morris, B.J.; le Couteur, D.G. Dietary approaches that delay age-related diseases. Clin. Interv. Aging 2006, 1, 11–31. [Google Scholar]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev 2009, 2, 270–278. [Google Scholar]
- Visioli, F.; Bogani, P.; Grande, S.; Galli, C. Mediterranean food and health: building human evidence. J. Physiol. Pharmacol 2005, 56, 37–49. [Google Scholar]
- Hulsmans, M.; Holvoet, P. The vicious circle between oxidative stress and inflammation in atherosclerosis. J. Cell. Mol. Med 2011, 14, 70–78. [Google Scholar]


| Human Study | Mechanisms involved | Authors’ conclusions |
|---|---|---|
| Landberg et al. | Endothelial dysfunction | Beneficial effects of dietary compounds, fruit, vegetables, fish and nuts, on endothelial dysfunction [29] |
| Kiecolt-Glaser, J.K. et al. | Telomere length and Inflammation | The lower n-6: n-3 (polyunsaturated fatty acid) PUFA ratios may be beneficial for slowing biological aging [30,31] |
| Weiss, E.P. et al. | Vascular aging | Studies in animals and humans indicate that caloric restriction prevent many of the age-related changes in the structure and function of the cardiovascular system [32] |
| Scoditti, E. et al. | Inflammation | Mediterranean diet polyphenols suppressed inflammatory angiogenesis [33] |
| Mirabello, L. et al. | Telomere length | A healthy lifestyle with a diet high in fruit and vegetables combined with exercise, lower body mass and not smoking is associated with longer telomeres [34] |
| Marin, C. et al. | Endothelial progenitor cell, microparticles, oxidative stress and telomere length | The Mediterranean diet is associated with improvement in endothelial regeneration capacity, increased number of circulating endothelial progenitors cell (EPC), lower levels of microparticles, reduce oxidative stress and decreased telomere shortening rate [35,36] |
| Fernandez, J.M. et al. | Endothelial progenitor cell | The consumption of a Mediterranean diet and exercise led a greater decrease in blood pressure and a greater increase in EPC number [37] |
| Martinez, P. et al. | Markers of oxidative stress | The Mediterranean diet reduces postprandial levels of oxidative stress biomarkers such as lipid peroxide, protein carbonyl, superoxide dismutase (SOD) activity and plasma H2O2 [38] |
| Cruz-Teno, C. et al. | Inflammatory state | The Mediterranean diet attenuates the postprandial inflammatory state, including nuclear transcription factor-kappa B (NF-κB), metalloproteinase-9 and tumor necrosis factor-α [39] |
| Cassidy, A. et al. | Telomere length | The dietary intake of fiber is positively correlated with leukocyte telomere length in women and negatively associated with dietary intake of polyunsaturated fatty acids, especially linoleic acid [40] |
| Farzaneh-Far, E. et al. Kielcolt-Glaser, J.K. et al. | Telomere shortening | In patients with coronary artery disease, there was an inverse relationship between baseline blood levels of marine omega-3 fatty acids and the rate of telomere shortening [31,41] |
| Yubero-Serrano, E.M. et al. | Oxidative stress | The Mediterranean diet, rich in virgin olive oil, induced a reduction in the degree of oxidative stress. In addition, coenzyme Q10 supplementation can improve antioxidant activity of cell membranes in the elderly [24,25] |
| Animal model study | Mechanisms involved | Author’s conclusion |
| Jung, K.J. et al. | Inflammation | Caloric restriction appears to attenuate vascular NF-κB induction and endothelial activation in aged rats[42,43] |
| McCarty, M.F. | Nitric oxide production | A low-fat, whole-food, vegan diet or exercise training would be expected to decrease the risk of common age-related diseases [26] |
| In vitro study | Mechanisms involved | Author’s conclusion |
| Csiszar, A. et al. | Mitochondria | Resveratrol induces mitochondrial biogenesis in cultured endothelial cells and in endothelia of mice with accelerated vascular aging [22,27] |
| Csiszar, A. et al. and Ungvari, Z. et al. | Inflammation and oxidative stress | In vitro studies suggest that the molecular mechanisms of resveratrol-mediated vasoprotection involve an inhibition of NF-κB and an upregulation of endothelial nitric oxide synthase (eNOS) and antioxidant enzymes [28,44–46] |
| Tang, Y. et al. | Cellular senescence and oxidative stress | In vitro studies suggest that resveratrol protects vascular cell senescence reducing the production of reactive oxygen species (ROS) [23,28] |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Marín, C.; Yubero-Serrano, E.M.; López-Miranda, J.; Pérez-Jiménez, F. Endothelial Aging Associated with Oxidative Stress Can Be Modulated by a Healthy Mediterranean Diet. Int. J. Mol. Sci. 2013, 14, 8869-8889. https://doi.org/10.3390/ijms14058869
Marín C, Yubero-Serrano EM, López-Miranda J, Pérez-Jiménez F. Endothelial Aging Associated with Oxidative Stress Can Be Modulated by a Healthy Mediterranean Diet. International Journal of Molecular Sciences. 2013; 14(5):8869-8889. https://doi.org/10.3390/ijms14058869
Chicago/Turabian StyleMarín, Carmen, Elena M Yubero-Serrano, José López-Miranda, and Francisco Pérez-Jiménez. 2013. "Endothelial Aging Associated with Oxidative Stress Can Be Modulated by a Healthy Mediterranean Diet" International Journal of Molecular Sciences 14, no. 5: 8869-8889. https://doi.org/10.3390/ijms14058869