Abstract
Background Under-treatment is frequently present in geriatric patients. Because this patient group often suffer from multiple diseases, polypharmacy (defined as the concomitant chronic use of five or more drugs) and contraindications to indicated drugs may also frequently be present.
Objective To describe the prevalence of under-treatment with respect to frequently indicated medications before and after comprehensive geriatric assessment (CGA) and the prevalence of contraindications to these medications.
Patients and Methods The geriatric outpatients evaluated in this study had previously been included in a prospective descriptive study conducted in 2004. Demographic data, medical history, co-morbidity and medication use and changes were documented. The absence of drugs indicated for frequently under-treated conditions before and after CGA was compared. Under-treatment was defined as omission of drug therapy indicated for the treatment or prevention of 13 established diseases or conditions known to be frequently under-treated. Co-morbid conditions were independently classified by two geriatricians, who determined whether or not a condition represented a contraindication to use of these drugs.
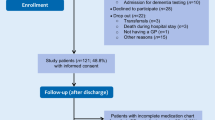
Results In 2004, 807 geriatric outpatients were referred for CGA. Of these, 548 patients had at least one of the 13 selected diseases or conditions. Thirty-two of these patients were excluded from the analysis, leaving 516 patients. Before CGA, 170 of these patients were under-treated (32.9%); after CGA, 115 patients (22.3%) were under-treated. Contraindications were present in 102 of the patients (19.8%) and were more frequent in under-treated patients. After CGA, mean drug use and the prevalence of polypharmacy increased. Although 393 drugs were discontinued after CGA, the overall number of drugs used increased from 3177 before CGA to 3424 after CGA. Five times more drugs were initiated for a new diagnosis than for correction of under-treatment.
Conclusions Under-treatment is significantly reduced after CGA. Patients with contraindications to indicated medicines are more frequently under-treated. CGA leads to an increase in polypharmacy, mainly because of new conditions being diagnosed and despite frequent discontinuation of medications.





Similar content being viewed by others
References
Rochon PA, Gurwitz JH. Prescribing for seniors: neither too much nor too little. JAMA 1999; 281(2): 113–5
Steinman MA, Landefeld CS, Rosenthal GE, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc 2006; 54: 1516–23
Denneboom W, Dautzenberg MGH, Grol R, et al. Analysis of polypharmacy in older patients in primary care using a multidisciplinary expert panel. Br J Gen Pract 2006; 56: 504–10
Kuijpers MA, van Marum RJ, Egberts AC, et al. Relationship between polypharmacy and underprescribing. Br J Clin Pharmacol 2008; 65(1): 130–3
Ko DT, Mamdani M, Alter DA. Lipid-lowering therapy with statins in high-risk elderly patients: the treatment-risk paradox. JAMA 2004; 291: 1864–70
Havranek EP, Abrams F, Stevens E, et al. Determinants of mortality in elderly patients with heart failure: the role of angiotensin-converting enzyme inhibitors. Arch Intern Med 1998; 158: 2024–8
Soumerai SB, McLaughlin TJ, Spiegelman D, et al. Adverse outcomes of underuse of beta-blockers in elderly survivors of acute myocardial infarction. JAMA 1997; 277: 115–21
Kuzuya M, Masuda Y, Hirakawa Y, et al. Underuse of medications for chronic disease in the oldest of community-dwelling older frail Japanese. J Am Geriatr Soc 2006; 54: 598–05
Komajda M, Follath F, Swedberg K, et al., on behalf of the Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology. The EuroHeart Failure Survey programme: a survey on the quality of care among patients with heart failure in Europe. Part 2: treatment. Eur Heart J 2003; 24: 464–74
Boyles PJ, Peterson GM, Bleasel MD, et al. Undertreatment of congestive heart failure in an Australian setting. J Clin Pharm Ther 2004; 29: 15–22
Tran CT, Laupacis A, Mamdani MM, et al. Effect of age on the use of evidence-based therapies for acute myocardial infarction. Am Heart J 2004; 148(5): 834–41
Higashi T, Shekelle PG, Solomon DH, et al. The quality of pharmacological care for vulnerable older patients. Ann Intern Med 2004; 140: 714–20
Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid conditions: implications for pay for performance. JAMA 2005; 294: 716–24
Baker DW, Hayes RP, Massie BM, et al. Variations in family physicians’ and cardiologists’ care for patients with heart failure. Am Heart J 1999; 138: 826–34
Lipton HL, Bero LA, Bird JA, et al. Undermedication among geriatric outpatients: results of a randomized controlled trial. Ann Rev Gerontol Ger 1992; 12: 95–108
Lee DS, Tu JV, Juurlink DN, et al. Risk-treatment mismatch in the pharmacotherapy of heart failure. JAMA 2005; 294: 1240–7
Schmader KE, Hanlon JT, Pieper CF, et al. Effects of geriatric evaluation and management on adverse drug reactions and suboptimal prescribing in the frail elderly. Am J Med 2004; 116: 394–401
Tulner LR, Frankfort SV, Gijsen GJ, et al. Drug-drug interactions in a geriatric outpatient cohort: prevalence and relevance. Drugs Aging 2008; 25(4): 343–55
Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–98
Meulen EF, Schmand B, van Campen JP, et al. The seven minute screen: a neurocognitive screening test highly sensitive to various types of dementia. J Neurol Neurosurg Psychiatry 2004; 75(5): 700–5
Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Maryland State Med J 1965; 14: 61–5
Fillenbaum GG. Screening the elderly: a brief instrumental activities of daily living measure. J Am Geriatr Soc 1985; 33: 698–706
Samsa GP, Hanlon JT, Schmader KE. A summated score for the Medication Appropriateness Index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol 1994; 47: 891–6
Charlson ME, Pompei P, Ales KA, et al. A new method of classifying prognostic comorbidity in longitudinal studies. J Chron Dis 1987; 40(5): 373–83
Dutch College of General Practitioners (Nederlands Huisartsen Genootschap). NHG-Standaarden [in Dutch; online]. Available from URL: http://nhg.artsennet.nl/kenniscentrum/k_richtlijnen/k_nhgstandaarden.htm [Accessed 2010 Aug 11]
Dutch Institute for Health Care Improvement, Medicines Evaluation Board, Dutch Ministry of Health, Welfare and Sport (College ter Beoordeling Geneesmiddelen, ministerie VWS). Database human medicines [in Dutch/English; online]. Available from URL: http://www.cbg-meb.nl/CBG/nl/humane-geneesmiddelen/geneesmiddeleninformatiebank/default.htm [Accessed 2010 Aug 11]
Limburg M, Tuut MK. CBO guideline ‘Stroke’ (revision) Dutch Institute for Healthcare Improvement [in Dutch]. Ned Tijdschr Geneeskd 2000 May 27; 144(22): 1058–62
Williams MA, Fleg JL, Ades A, et al. Secondary prevention of coronary heart disease in the elderly (with emphasis on patients ≥75 years of age): an American Heart Association scientific statement from the Council on Clinical Cardiology Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention. Circulation 2002; 105: 1735–43
Frankfort SV, Tulner CR, van Campen JPCM, et al. Evaluation of pharmacotherapy in geriatric patients after performing complete geriatric assessment (CGA) at a diagnostic day clinic. Clin Drug Invest 2006; 26: 169–74
Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA 2003; 290: 2685–92
Man-Son-Hing M, Nichol G, Lau A, et al. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999; 159: 677–85
Bond AJ, Molnar FJ, Li M, et al. The risk of hemorrhagic complications in hospital in-patients who fall while receiving antithrombotic therapy. Thrombosis J 2005; 3: 1 [online]. Available from URL: http://www.thrombosisjournal.com/content/3/1/1 [Accessed 2010 Aug 24]
Gage BF, Birman Deych E, Kerzner R, et al. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med 2005; 118: 612–7
Ueno H, Ohshita T, Kondo K, et al. Association between microbleeds on T3*-weighted images and recurrent hemorrhagic stroke in patients treated with warfarin following ischemic stroke. Am J Neuroradiol 2008; 29(8): 1483–6
Lee SH, Ryu WS, Roh JK. Cerebral microbleeds are a risk factor for warfarin related intracerebral hemorrhage. Neurology 2009; 72: 171–6
Cordonnier C, van der Flier WM, Sluimer JD, et al. Prevalence and severity of microbleeds in a memory clinic setting. Neurology 2006; 66: 1356–60
van Deelen BAJ, van den Beemt PMLA, Egberts TCG, et al. Cognitive impairment as determinant for sub-optimal control of oral anticoagulation treatment in elderly patients with atrial fibrillation. Drugs Aging 2005; 22(4): 353–60
Kagansky N, Knobler H, Rimon E, et al. Safety of anticoagulation therapy in well-informed older patients. Arch Intern Med 2004; 164: 2044–50
AD 2000 Collaborative group, Bentham P, Gray R, et al. Aspirin in Alzheimer’s disease: a randomized open-label trial. Lancet Neurol 2008; 7(1): 41–9
Mant J, Hobbs R, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged study, BAFTA): a randomised controlled trial. Lancet 2007; 370: 493–503
Goldberger JJ, Bonow RO, Cuffe M, et al. Post-myocardial infarction β-blocker therapy — the bradycardia conundrum: rationale and design for the Pacemaker and β-blocker therapy post-MI (PACE-MI) trial. Am Heart J 2008; 155: 455–64
Andrus MR, Loyed JV. Use of β-adrenoreceptor antagonists in older patients with chronic obstructive pulmonary disease and cardiovascular co-morbidity: safety issues. Drugs Aging 2008; 25(2): 131–44
Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Long-term compliance with beta blockers, angiotensinconverting enzyme inhibitors, and statins after acute myocardial infarction. Eur Heart J 2006; 27: 1153–8
Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool for Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment): consensus validation. Int J Clin Pharmacol Ther 2008; 46: 72–83
Ryan C, O’Mahony D, Kennedy J, et al. Potentially inappropriate prescribing in an Irish elderly population in primary care. Br J Clin Pharmacol 2009; 68(6): 936–47
Drenth-van Maanen AC, van Marum RJ, Knol W, et al. Prescribing optimization method for improving prescribing in elderly patients receiving polypharmacy: results of application to case histories by general practitioners. Drugs Aging 2009; 26(8): 687–701
Acknowledgements
No sources of funding were used to conduct this study or prepare this manuscript. The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tulner, L.R., van Campen, J.P.C.M., Frankfort, S.V. et al. Changes in Under-Treatment after Comprehensive Geriatric Assessment. Drugs Aging 27, 831–843 (2010). https://doi.org/10.2165/11539330-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11539330-000000000-00000