Abstract
Background and Objective
Midazolam is used to sedate children during extracorporeal membrane oxygenation (ECMO). Pharmacokinetic changes are expected because of extracorporeal circulation and maturation. We present a population pharmacokinetic model for midazolam and its major metabolites in neonates during venoarterial ECMO.
Methods
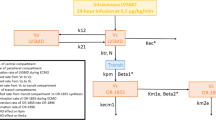
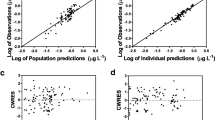
We studied 20 neonates on venoarterial ECMO, with a median postnatal age of 0.79 (range 0.17–5.8) days and a bodyweight of 3.0 (range 2.7–3.9) kg at the onset of ECMO. The median ECMO duration was 124 (range 70–275) hours. Serum concentrations were measured at the initiation and discontinuation of the midazolam infusion (100–300 μg/kg/h). Analysis of concentrations of midazolam, 1-hydroxymidazolam and its glucuronide were performed using nonlinear mixed-effects modelling. A two-compartment model for midazolam and a one-compartment model for the metabolites 1-hydroxymidazolam and hydroxymidazolam glucuronide adequately described the data, with allometric scaling of all parameters.
Results
Following the start of ECMO, the volume of distribution of midazolam increased from 4.29 to 14.6 L/3kg, with an elimination half-life of 1.85 hours. The median midazolam and 1-hydroxymidazolam clearance values increased 3-fold within the first 5 days (up to 1.38 and 5.31 L/h/3 kg, respectively), whereas hydroxymidazolam glucuronide clearance remained constant at 0.18 L/h/3 kg. Interpatient variability estimates of midazolam, 1-hydroxymidazolam and hydroxymidazolam glucuronide clearance and midazolam and hydroxymidazolam glucuronide volumes of distribution varied between 87% and 129%. Concomitant inotropic infusion increased hydroxymidazolam glucuronide clearance by 23%.
Conclusion
After allometric scaling, clearance of midazolam and 1-hydroxymidazolam increases as a result of maturation or recovery from critical illness. In ECMO patients weighing 2.7–3.9 kg, continuously infused midazolam doses of 300 μg/kg/h for 6 hours and 150 μg/kg/h thereafter provide adequate serum concentrations for sedation. The dose must be increased substantially after 5–7 days. Hydroxymidazolam glucuronide accumulates during ECMO, providing an increased proportion of the overall effect, up to 34% after 7 days. Large unexplained interpatient variability warrants careful titration of sedation and adverse effects.








Similar content being viewed by others
References
Jacqz-Aigrain E, Daoud P, Burtin P, et al. Pharmacokinetics of midazolam during continuous infusion in critically ill neonates. Eur J Clin Pharmacol 1992; 42(3): 329–32
Buck ML. Pharmacokinetic changes during extracorporeal membrane oxygenation: implications for drug therapy of neonates. Clin Pharmacokinet 2003; 42(5): 403–17
Dagan O, Klein J, Gruenwald C, et al. Preliminary studies of the effects of extracorporeal membrane oxygenator on the disposition of common pediatric drugs. Ther Drug Monit 1993 Aug; 15(4): 263–6
Mulla H, Lawson G, von Anrep C, et al. In vitro evaluation of sedative drug losses during extracorporeal membrane oxygenation. Perfusion 2000 Jan; 15(1): 21–6
Martens HJ, De Goede PN, Van Loenen AC. Sorption of various drugs in polyvinyl chloride, glass, and polyethylene-lined infusion containers. Am J Hosp Pharm 1990 Feb; 47(2): 369–73
Mulla H, Lawson G, Woodland ED, et al. Effects of neonatal extracorporeal membrane oxygenation circuits on drug disposition. Curr Ther Res 2000; 61(11): 838–48
Mulla H, McCormack P, Lawson G, et al. Pharmacokinetics of midazolam in neonates undergoing extracorporeal membrane oxygenation. Anesthesiology 2003 Aug; 99(2): 275–82
Zhu B, Bush D, Doss GA, et al. Characterization of 1′-hydroxymidazolam glucuronidation in human liver microsomes. Drug Metab Dispos 2008 Feb; 36(2): 331–8
Stevens JC. New perspectives on the impact of cytochrome P450 3A expression for pediatric pharmacology. Drug Discov Today 2006 May; 11(9–10): 440–5
Kearns GL, Abdel-Rahman SM, Alander SW, et al. Developmental pharmacology: drug disposition, action, and therapy in infants and children. N Engl J Med 2003 Sep 18; 349(12): 1157–67
Mandema JW, Tuk B, van Steveninck AL, et al. Pharmacokinetic-pharmacodynamic modeling of the central nervous system effects of midazolam and its main metabolite alpha-hydroxymidazolam in healthy volunteers. Clin Pharmacol Ther 1992 Jun; 51(6): 715–28
Bauer TM, Ritz R, Haberthur C, et al. Prolonged sedation due to accumulation of conjugated metabolites of midazolam. Lancet 1995 Jul 15; 346(8968): 145–7
van Dijk M, Peters JW, van Deventer P, et al. The COMFORT Behavior Scale: a tool for assessing pain and sedation in infants. Am J Nurs 2005 Jan; 105(1): 33–6
Ista E, van Dijk M, Tibboel D, et al. Assessment of sedation levels in pediatric intensive care patients can be improved by using the COMFORT ‘Behavior’ Scale. Pediatr Crit Care Med 2005 Jan; 6(1): 58–63
Peeters MY, Prins SA, Knibbe CA, et al. Pharmacokinetics and pharmacodynamics of midazolam and metabolites in nonventilated infants after craniofacial surgery. Anesthesiology 2006 Dec; 105(6): 1135–46
Jonsson EN, Karlsson MO. Xpose: an S-Plus based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 1999 Jan; 58(1): 51–64
Mandema JW, Verotta D, Sheiner LB. Building population pharmacokineticpharmacodynamic models: I. Models for covariate effects. J Pharmacokinet Biopharm 1992 Oct; 20(5): 511–28
Anderson BJ, Allegaert K, Holford NH. Population clinical pharmacology of children: modelling covariate effects. Eur J Pediatr 2006 Dec; 165(12): 819–29
de Wildt SN, Kearns GL, Leeder JS, et al. Cytochrome P450 3A: ontogeny and drug disposition. Clin Pharmacokinet 1999 Dec; 37(6): 485–505
de Wildt SN, Kearns GL, Leeder JS, et al. Glucuronidation in humans: pharmacogenetic and developmental aspects. Clin Pharmacokinet 1999 Jun; 36(6): 439–52
Zuppa AF, Nadkarni V, Davis L, et al. The effect of a thyroid hormone infusion on vasopressor support in critically ill children with cessation of neurologic function. Crit Care Med 2004 Nov; 32(11): 2318–22
Ette EI. Stability and performance of a population pharmacokinetic model. J Clin Pharmacol 1997 Jun; 37(6): 486–95
Meibohm B, Laer S, Panetta JC, et al. Population pharmacokinetic studies in pediatrics: issues in design and analysis. AAPS J 2005; 7(2): E475–87
Bjorkman S. Prediction of cytochrome P450-mediated hepatic drug clearance in neonates, infants and children: how accurate are available scaling methods?. Clin Pharmacokinet 2006; 45(1): 1–11
Anderson BJ, McKee AD, Holford NH. Size, myths and the clinical pharmacokinetics of analgesia in paediatric patients. Clin Pharmacokinet 1997 Nov; 33(5): 313–27
Burtin P, Jacqz-Aigrain E, Girard P, et al. Population pharmacokinetics of midazolam in neonates. Clin Pharmacol Ther 1994 Dec; 56(6 Pt 1): 615–25
de Wildt SN, Kearns GL, Hop WC, et al. Pharmacokinetics and metabolism of intravenous midazolam in preterm infants. Clin Pharmacol Ther 2001 Dec; 70(6): 525–31
de Wildt SN, de Hoog M, Vinks AA, et al. Population pharmacokinetics and metabolism of midazolam in pediatric intensive care patients. Crit Care Med 2003 Jul; 31(7): 1952–8
Hartwig S, Roth B, Theisohn M. Clinical experience with continuous intravenous sedation using midazolam and fentanyl in the paediatric intensive care unit. Eur J Pediatr 1991 Sep; 150(11): 784–8
Lacroix D, Sonnier M, Moncion A, et al. Expression of CYP3A in the human liver: evidence that the shift between CYP3A7 and CYP3A4 occurs immediately after birth. Eur J Biochem 1997 Jul 15; 247(2): 625–34
Daly AK. Significance of the minor cytochrome P450 3A isoforms. Clin Pharmacokinet 2006; 45(1): 13–31
Williams JA, Ring BJ, Cantrell VE, et al. Comparative metabolic capabilities of CYP3A4, CYP3A5, and CYP3A7. Drug Metab Dispos 2002 Aug; 30(8): 883–91
Fragen RJ. Pharmacokinetics and pharmacodynamics of midazolam given via continuous intravenous infusion in intensive care units. Clin Ther 1997 May–Jun; 19(3): 405–19; discussion 367-8
Peters JW, Anderson BJ, Simons SH, et al. Morphine metabolite pharmacokinetics during venoarterial extra corporeal membrane oxygenation in neonates. Clin Pharmacokinet 2006; 45(7): 705–14
Smith JC, Davies MC, Melia CD, et al. Uptake of drugs by catheters: the influence of the drug molecule on sorption by polyurethane catheters. Biomaterials 1996 Aug; 17(15): 1469–72
Mehta NM, Halwick DR, Dodson BL, et al. Potential drug sequestration during extracorporeal membrane oxygenation: results from an ex vivo experiment. Intensive Care Med 2007 Jun; 33(6): 1018–24
Bhatt-Meht V, Annich G. Sedative clearance during extracorporeal membrane oxygenation. Perfusion 2005 Oct; 20(6): 309–15
Goldstein SL. Kidney function assessment in the critically ill child: is it time to leave creatinine behind?. Crit Care 2007; 11(3): 141
Harrison AM, Davis S, Eggleston S, et al. Serum creatinine and estimated creatinine clearance do not predict perioperatively measured creatinine clearance in neonates undergoing congenital heart surgery. Pediatr Crit Care Med 2003 Jan; 4(1): 55–9
Hughes J, Gill AM, Mulhearn H, et al. Steady-state plasma concentrations of midazolam in critically ill infants and children. Ann Pharmacother 1996 Jan; 30(1): 27–30
Acknowledgements
The authors thank Saskia N. de Wildt, MD, PhD, for her critical evaluation of this manuscript. This study was funded solely by institutional funding. The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahsman, M.J., Hanekamp, M., Wildschut, E.D. et al. Population Pharmacokinetics of Midazolam and Its Metabolites during Venoarterial Extracorporeal Membrane Oxygenation in Neonates. Clin Pharmacokinet 49, 407–419 (2010). https://doi.org/10.2165/11319970-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11319970-000000000-00000