Abstract
Treatment effects are often evaluated by comparing change over time in outcome measures. However, valid analyses of longitudinal data can be problematic when subjects discontinue (dropout) prior to completing the trial. This study compared the Type I error rates from a likelihood-based repeated measures analysis (MMRM) to a fixed-effects analysis of variance where missing values were imputed using the last observation carried forward approach (LOCF). Comparisons were made in 32 scenarios, with 3000 simulated data sets per scenario. The null hypothesis of no difference between treatments in mean change from baseline to endpoint was true in all data sets. Subject dropout was introduced to generate ignorable and nonignorable missingness.
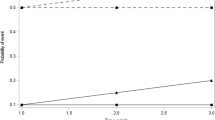
Pooled across all scenarios, the Type I error rates for MMRM and LOCF were 5.85% and 10.36%, respectively. Type I error rates in the 32 scenarios ranged from 5.03% to 7.17% for MMRM, and from 4.43% to 36.30% for LOCF. In 19 of the 32 scenarios, MMRM yielded a Type I error rate that was at least 1.00% closer to the expected rate of 5.00% than the corresponding rate from LOCF.
Greater inflation of Type I error in LOCF resulted from greater bias in estimates of mean change from baseline to endpoint and unduly small standard errors that were a consequence of failing to account for the uncertainty of imputation. The superior control of Type I error by MMRM suggested that MMRM should replace LOCF as the default primary analysis for longitudinal clinical trials where dropout bias may exist.
Similar content being viewed by others
References
Milliken GA, Johnson DE. “The Analysis of Messy Data.” In Vol. I. Designed Experiments. New York, NY: Chapman & Hall; 1993:326.
Gibbons RD, Hedeker D, Elkin I, Waternaux C, Kraemer HC, Greenhouse JB, Shea T, Imber S, Stotsky SM, Watkins JT. Some conceptual and statistical issues in analysis of longitudinal psychiatric data. Arch Gen Psych. 1993;50:739–750.
Laird NM. Missing data in longitudinal studies. Stat Med. 1988;7:305–315.
Little R, Rubin D. Statistical Analysis with Missing Data. New York, NY: John Wiley and Sons: 1987.
Lavori PW. Clinical trials in psychiatry: Should protocol deviation censor patient data? Neuropsycho-pharmacol. 1992;6(1):39–48.
Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York, NY: Springer; 2000.
Littell RC, Milliken GA, Stroup WW, Wolfinger RD. The SAS System for Mixed Models. Cary, NC: SAS Institute Inc; 1996; Chap. 1–10.
Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyze unbalanced repeated measures and longitudinal data. Stat Med. 1997; 16: 2349–2380.
Lavori PW, Dawson R, Shera D. A multiple imputation strategy for clinical trials with truncation of patient data. Stat Med. 1995;14:1913–1925.
Siddiqui O, Ali MW. A comparison of the random-effects pattern mixture model with last-observation-carried-forward (LOCF) analysis in longitudinal clinical trials with dropouts. J Biopharm Stat. 1998;8(4):545–563.
Heyting A, Tolboom JTBM, Essers JGA. Statistical handling of dropouts in longitudinal clinical trials. Stat Med. 1992;11:2043–2061.
Mallinckrodt CH, Clark WS, David SR. Accounting for dropout bias using mixed-effects models. J Bio-Pharm Stat. Forthcoming.
Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda, NY: Multi-Health Systems; 1986.
Tollefson GD, Beasley CM, Jr., Tran P, Street JS, Krueger JA, Tamura RN, Graffeo KA, Thieme ME. Olanzapine versus Haloperidol in the treatment of schizophrenia, schizoaffective, and schizophreniform disorders: Results of an international collaborative trial. Am J Psychiatry. 1997;154(4):457–465.
Van Vleck LD, Gregory KE. Multiple trait restricted maximum likelihood for simulated measures of ovulation rate with underlying multivariate normal distributions. J Anim Sci. 1992;70:57.
Mallinckrodt CH. The Effects of Animal Model Approximations and Data Problems on the Reliability of Genetic Evaluations. Ph.D. Dissertation. Fort Collins, CO: Colorado State University; 1993.
Littell RC, Milliken GA, Stroup WW, Wolfinger RD. The SAS System for Mixed Models. Cary, NC: SAS Institute Inc; 1996;499.
Littell RC, Milliken GA, Stroup WW, Wolfinger RD. The SAS System for Mixed Models. Cary, NC: SAS Institute Inc; 1996;38.
Rubin D, Shenker N. Multiple imputations in health care databases: An overview and some applications. Stat Med. 1991;10:585–598.
Little R, Yau L. Intent-to-treat analysis for longitudinal studies with drop-outs. Biometrics. 1996;52: 1324–1333.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mallinckrodt, C.H., Clark, W.S. & David, S.R. Type I Error Rates from Mixed Effects Model Repeated Measures Versus Fixed Effects Anova with Missing Values Imputed Via Last Observation Carried Forward. Ther Innov Regul Sci 35, 1215–1225 (2001). https://doi.org/10.1177/009286150103500418
Published:
Issue Date:
DOI: https://doi.org/10.1177/009286150103500418