Key Points
-
Myeloid-cell activation is controlled by many families of receptors, each including activating and inhibitory isoforms.
-
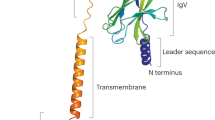
Triggering receptors expressed by myeloid cells (TREMs) are encoded by a gene cluster on human chromosome 6 and mouse chromosome 17.
-
TREM1 is associated with the DAP12 adaptor and amplifies granulocytic and monocytic inflammatory responses during bacteria and fungus infections.
-
Blocking TREM1 function reduces mortality in experimental models of sepsis.
-
TREM2 can associate with the DAP12 adaptor and activates monocyte-derived dendritic cells.
-
TREM2 promotes the differentiation of osteoclasts and glial cells from monocytic precursors.
-
Rare null mutations of TREM2 in humans cause Nasu-Hakola disease, an autosomic recessive disorder that is characterized by bone cysts and demyelination of the central nervous system, resulting in bone fractures and presenile dementia.
Abstract
Triggering receptors expressed by myeloid cells (TREMs) belong to a rapidly expanding family of receptors that include activating and inhibitory isoforms encoded by a gene cluster linked to the MHC. TREM1 and TREM2 activate myeloid cells by signalling through the adaptor protein DAP12. TREM1 triggers phagocyte secretion of pro-inflammatory chemokines and cytokines, amplifying the inflammation that is induced by bacteria and fungi. TREM2 activates monocyte-derived dendritic cells and regulates osteoclast development. Remarkably, TREM2 deficiency leads to a severe disease that is characterized by bone cysts and demyelination of the central nervous system, which results in dementia, implying that the function of TREM2 extends beyond the immune system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hoffmann, J. A., Kafatos, F. C., Janeway, C. A. & Ezekowitz, R. A. Phylogenetic perspectives in innate immunity. Science 284, 1313–1318 (1999).
Medzhitov, R. Toll-like receptors and innate immunity. Nature Rev. Immunol. 1, 135–145 (2001).
Poltorak, A. et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998).
Akira, S., Takeda, K. & Kaisho, T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nature Immunol. 2, 675–680 (2001).
Aderem, A. & Underhill, D. M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17, 593–623 (1999).
Linehan, S. A., Martinez-Pomares, L. & Gordon, S. Mannose receptor and scavenger receptor: two macrophage pattern recognition receptors with diverse functions in tissue homeostasis and host defense. Adv. Exp. Med. Biol. 479, 1–14 (2000).
Brown, G. D. et al. Dectin-1 is a major β-glucan receptor on macrophages. J. Exp. Med. 196, 407–412 (2002).
Barrington, R., Zhang, M., Fischer, M. & Carroll, M. C. The role of complement in inflammation and adaptive immunity. Immunol. Rev. 180, 5–15 (2001).
Fraser, I. P., Koziel, H. & Ezekowitz, R. A. The serum mannose-binding protein and the macrophage mannose receptor are pattern recognition molecules that link innate and adaptive immunity. Semin. Immunol. 10, 363–372 (1998).
Lanier, L. L. NK cell receptors. Annu. Rev. Immunol. 16, 359–393 (1998).
Dietrich, J., Nakajima, H. & Colonna, M. Human inhibitory and activating Ig-like receptors which modulate the function of myeloid cells. Microbes Infect. 2, 323–329 (2000).
Oldenborg, P. A. et al. Role of CD47 as a marker of self on red blood cells. Science 288, 2051–2054 (2000).
Wright, G. J. et al. Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity 13, 233–242 (2000).
Hoek, R. M. et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science 290, 1768–1771 (2000).
Barclay, A. N., Wright, G. J., Brooke, G. & Brown, M. H. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. 23, 285–290 (2002).
Crocker, P. R. & Varki, A. Siglecs, sialic acids and innate immunity. Trends Immunol. 22, 337–342 (2001).
Trowsdale, J. Genetic and functional relationships between MHC and NK receptor genes. Immunity 15, 363–374 (2001).
Tomasello, E., Blery, M., Vely, E. & Vivier, E. Signaling pathways engaged by NK cell receptors: double concerto for activating receptors, inhibitory receptors and NK cells. Semin. Immunol. 12, 139–147 (2000).
Arase, H., Mocarski, E. S., Campbell, A. E., Hill, A. B. & Lanier, L. L. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296, 1323–1326 (2002).
Smith, H. R. et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc. Natl Acad. Sci. USA 99, 8826–8831 (2002).
Bauer, S. et al. Activation of NK cells and T cells by NKG2D, a receptor for stress- inducible MICA. Science 285, 727–729 (1999).
Diefenbach, A., Jamieson, A. M., Liu, S. D., Shastri, N. & Raulet, D. H. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nature Immunol. 1, 119–126 (2000).
Cerwenka, A. et al. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity 12, 721–727 (2000).
Bouchon, A., Dietrich, J. & Colonna, M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 164, 4991–4995 (2000). This paper reported the original cloning of the triggering receptors expressed by myeloid cells (TREMs).
Bouchon, A., Hernandez-Munain, C., Cella, M. & Colonna, M. A DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. J. Exp. Med. 194, 1111–1122 (2001). A paper that characterized the function of TREM2 in dendritic cells.
McVicar, D. W. et al. DAP12-mediated signal transduction in natural killer cells. A dominant role for the Syk protein-tyrosine kinase. J. Biol. Chem. 273, 32934–32942 (1998).
Gingras, M. C., Lapillonne, H. & Margolin, J. F. TREM-1, MDL-1, and DAP12 expression is associated with a mature stage of myeloid development. Mol. Immunol. 38, 817–824 (2002).
Daws, M. R., Lanier, L. L., Seaman, W. E. & Ryan, J. C. Cloning and characterization of a novel mouse myeloid DAP12-associated receptor family. Eur. J. Immunol. 31, 783–791 (2001). The first group to characterize mouse Trems.
Chung, D. H., Seaman, W. E. & Daws, M. R. Characterization of TREM-3, an activating receptor on mouse macrophages: definition of a family of single Ig domain receptors on mouse chromosome 17. Eur. J. Immunol. 32, 59–66 (2002).
Cantoni, C. et al. NKp44, a triggering receptor involved in tumor cell lysis by activated human natural killer cells, is a novel member of the immunoglobulin superfamily. J. Exp. Med. 189, 787–796 (1999).
Jackson, D. G., Hart, D. N., Starling, G. & Bell, J. I. Molecular cloning of a novel member of the immunoglobulin gene superfamily homologous to the polymeric immunoglobulin receptor. Eur. J. Immunol. 22, 1157–1163 (1992).
Green, B. J., Clark, G. J. & Hart, D. N. The CMRF-35 mAb recognizes a second leukocyte membrane molecule with a domain similar to the poly Ig receptor. Int. Immunol. 10, 891–899 (1998).
Speckman, R. A. et al. Novel immunoglobulin superfamily gene cluster, mapping to a region of human chromosome 17q25, linked to psoriasis susceptibility. Hum. Genet. 112, 34–41 (2003).
Wu, J., Cherwinski, H., Spies, T., Philips, J. H. & Lanier, L. L. DAP10 and DAP12 form distinct, but functionally cooperative, receptor complexes in natural killer cells. J. Exp. Med. 192, 1059–1068 (2000).
Allcock, R. J., Barrow, A. D., Forbes, S., Beck, S. & Trowsdale, J. The human TREM gene cluster at 6p21.2 encodes both activating and inhibitory single IgV domain receptors and includes NKp44. Eur. J. Immunol. 33, 567–577 (2003).
Washington, A. V., Quigley, L. & McVicar, D. W. Initial characterization of TREM-like transcript (TLT)-1: a putative inhibitory receptor within the TREM cluster. Triggering receptors expressed on myeloid cells. Blood 100, 3822–3824 (2002).
Colonna, M. & Facchetti, F. TREM-1: a new player in acute inflammatory responses. J. Infect. Dis. (in the press).
Bouchon, A., Facchetti, F., Weigand, M. A. & Colonna, M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 410, 1103–1107 (2001). Identification of the pro-inflammatory function of TREM1.
Bleharski, J. R. et al. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J. Immunol. 170, 3812–3818 (2003).
Lucas, M. et al. Massive inflammatory syndrome and lymphocytic immunodeficiency in KARAP/DAP12-transgenic mice. Eur. J. Immunol. 32, 2653–2663 (2002).
Wang, H. et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285, 248–251 (1999).
Bernhagen, J. et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature 365, 756–759 (1993).
Tracey, K. J. & Abraham, E. From mouse to man: or what have we learned about cytokine-based anti-inflammatory therapies? Shock 11, 224–225 (1999).
Ware, L. B. & Matthay, M. A. The acute respiratory distress syndrome. N. Engl. J. Med. 342, 1334–1349 (2000).
Banchereau, J. & Steinman, R. M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Regnault, A. et al. Fcγ receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 189, 371–380 (1999).
Tomasello, E. et al. Combined natural killer cell and dendritic cell functional deficiency in KARAP/DAP12 loss-of-function mutant mice. Immunity 13, 355–364 (2000).
Bakker, A. B. et al. DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming. Immunity 13, 345–353 (2000).
Dietrich, J., Cella, M., Seiffert, M., Buhring, H. J. & Colonna, M. Cutting edge: signal-regulatory protein β1 is a DAP12-associated activating receptor expressed in myeloid cells. J. Immunol. 164, 9–12 (2000).
Hakola, H. P., Jarvi, O. H. & Sourander, P. Osteodysplasia polycystica hereditaria combined with sclerosing leucoencephalopathy, a new entity of the dementia praesenilis group. Acta Neurol. Scand. 46, S79 (1970).
Nasu, T., Tsukahara, Y. & Terayama, K. A lipid metabolic disease — 'membranous lipodystrophy' — an autopsy case demonstrating numerous peculiar membrane-structures composed of compound lipid in bone and bone marrow and various adipose tissues. Acta Pathol. Jpn 23, 539–558 (1973).
Verloes, A. et al. Nasu-Hakola syndrome: polycystic lipomembranous osteodysplasia with sclerosing leucoencephalopathy and presenile dementia. J. Med. Genet. 34, 753–757 (1997).
Kitajima, I. et al. Nasu-Hakola disease (membranous lipodystrophy). Clinical, histopathological and biochemical studies of three cases. J. Neurol. Sci. 91, 35–52 (1989).
Pekkarinen, P. et al. Assignment of the locus for PLO-SL, a frontal-lobe dementia with bone cysts, to 19q13. Am. J. Hum. Genet. 62, 362–372 (1998).
Pekkarinen, P. et al. Fine-scale mapping of a novel dementia gene, PLOSL, by linkage disequilibrium. Genomics 54, 307–315 (1998).
Paloneva, J. et al. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nature Genet. 25, 357–361 (2000).
Kondo, T. et al. Heterogeneity of presenile dementia with bone cysts (Nasu-Hakola disease): Three genetic forms. Neurology 59, 1105–1107 (2002).
Kaifu, T. et al. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J. Clin. Invest. 111, 323–332 (2003). This study characterizes the role of DAP12 in the development of osteoclasts and oligodendrocytes in vivo and in vitro.
Paloneva, J. et al. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am. J. Hum. Genet. 71, 656–662 (2002). Original identification of TREM2 mutations as the basis of Nasu-Hakola disease.
Teitelbaum, S. L. Bone resorption by osteoclasts. Science 289, 1504–1508 (2000).
Wong, B. R., Josien, R. & Choi, Y. TRANCE is a TNF family member that regulates dendritic cell and osteoclast function. J. Leukocyte Biol. 65, 715–724 (1999).
Schmid, C. D. et al. Heterogeneous expression of the triggering receptor expressed on myeloid cells-2 on adult murine microglia. J. Neurochem. 83, 1309–1320 (2002).
Kreutzberg, G. W. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 19, 312–318 (1996).
Daws, M. R., Sullam, P. M., Niemi, E. C., Chen, T. T. & Seaman, W. E. Pattern recognition by TREM-2: binding of anionic ligands. J. Immunol. (in the press). The first identification of a TREM2 ligand.
Aoki, N. et al. The role of the DAP12 signal in mouse myeloid differentiation. J. Immunol. 165, 3790–3796 (2000).
Aoki, N. et al. DAP12 ITAM motif regulates differentiation and apoptosis in M1 leukemia cells. Biochem. Biophys. Res. Commun. 291, 296–304 (2002).
Samaridis, J. & Colonna, M. Cloning of novel immunoglobulin superfamily receptors expressed on human myeloid and lymphoid cells: structural evidence for new stimulatory and inhibitory pathways. Eur. J. Immunol. 27, 660–665 (1997).
Colonna, M. et al. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J. Exp. Med. 186, 1809–1818 (1997).
Cosman, D. et al. A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity 7, 273–282 (1997).
Colonna, M. et al. Human myelomonocytic cells express an inhibitory receptor for classical and nonclassical MHC class I molecules. J. Immunol. 160, 3096–3100 (1998).
Borges, L., Hsu, M. L., Fanger, N., Kubin, M. & Cosman, D. A family of human lymphoid and myeloid Ig-like receptors, some of which bind to MHC class I molecules. J. Immunol. 159, 5192–5196 (1997).
Allen, R. L., Raine, T., Haude, A., Trowsdale, J. & Wilson, M. J. Leukocyte receptor complex-encoded immunomodulatory receptors show differing specificity for alternative HLA-B27 structures. J. Immunol. 167, 5543–5547 (2001).
Nakajima, H., Samaridis, J., Angman, L. & Colonna, M. Human myeloid cells express an activating ILT receptor (ILT1) that associates with Fc receptor γ-chain. J. Immunol. 162, 5–8 (1999).
Kubagawa, H., Burrows, P. D. & Cooper, M. D. A novel pair of immunoglobulin-like receptors expressed by B cells and myeloid cells. Proc. Natl Acad. Sci. USA 94, 5261–5266 (1997).
Kharitonenkov, A. et al. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature 386, 181–186 (1997).
Brown, E. J. & Frazier, W. A. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 11, 130–135 (2001).
Oldenborg, P. A. et al. Role of CD47 as a marker of self on red blood cells. Science 288, 2051–2054 (2000).
Dietrich, J., Cella, M., Seiffert, M., Buhring, H. J. & Colonna, M. Cutting edge: signal-regulatory protein β1 is a DAP12-associated activating receptor expressed in myeloid cells. J. Immunol. 164, 9–12 (2000).
Tomasello, E. et al. Association of signal-regulatory proteins β with KARAP/DAP-12. Eur. J. Immunol. 30, 2147–2156 (2000).
Wright, G. J. et al. Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity 13, 233–242 (2000).
Hoek, R. M. et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science 290, 1768–1771 (2000).
Barclay, A. N., Wright, G. J., Brooke, G. & Brown, M. H. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. 23, 285–290 (2002).
Fournier, N. et al. FDF03, a novel inhibitory receptor of the immunoglobulin superfamily, is expressed by human dendritic and myeloid cells. J. Immunol. 165, 1197–1209 (2000).
Mousseau, D. D., Banville, D., L'Abbe, D., Bouchard, P. & Shen, S. H. PILRα, a novel immunoreceptor tyrosine-based inhibitory motif-bearing protein, recruits SHP-1 upon tyrosine phosphorylation and is paired with the truncated counterpart PILRβ. J. Biol. Chem. 275, 4467–4474 (2000).
Cantoni, C. et al. Molecular and functional characterization of IRp60, a member of the immunoglobulin superfamily that functions as an inhibitory receptor in human NK cells. Eur. J. Immunol. 29, 3148–3159 (1999).
Bakker, A. B., Baker, E., Sutherland, G. R., Phillips, J. H. & Lanier, L. L. Myeloid DAP12-associating lectin (MDL)-1 is a cell surface receptor involved in the activation of myeloid cells. Proc. Natl Acad. Sci. USA 96, 9792–9796 (1999).
Acknowledgements
I would like to thank S. Gilfillan and W. Barchet for helpful comments.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
LocusLink
OMIM
FURTHER INFORMATION
Glossary
- MICROGLIA
-
Bone-marrow-derived macrophage lineage cells that are present in the CNS.
- CAECAL LIGATION AND PUNCTURE
-
An experimental model of polymicrobial sepsis that is induced by ligation and perforation of the caecum.
Rights and permissions
About this article
Cite this article
Colonna, M. TREMs in the immune system and beyond. Nat Rev Immunol 3, 445–453 (2003). https://doi.org/10.1038/nri1106
Issue Date:
DOI: https://doi.org/10.1038/nri1106
This article is cited by
-
Recent advances on the molecular mechanisms of exercise-induced improvements of cognitive dysfunction
Translational Neurodegeneration (2023)
-
Translational molecular imaging and drug development in Parkinson’s disease
Molecular Neurodegeneration (2023)
-
Title: Bioinformatic Identification of Genes Involved in Diabetic Nephropathy Fibrosis and their Clinical Relevance
Biochemical Genetics (2023)
-
The orchestrating role of deteriorating neurons and TREM-1 in crosstalk with SYK in Alzheimer’s disease progression and neuroinflammation
Inflammopharmacology (2023)
-
Role of TREM2 in the Development of Neurodegenerative Diseases After Traumatic Brain Injury
Molecular Neurobiology (2023)