Abstract
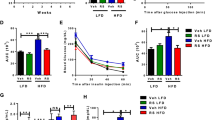
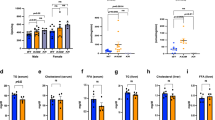
Obesity induced in mice by high-fat feeding activates the protein kinase Cdk5 (cyclin-dependent kinase 5) in adipose tissues. This results in phosphorylation of the nuclear receptor PPARγ (peroxisome proliferator-activated receptor γ), a dominant regulator of adipogenesis and fat cell gene expression, at serine 273. This modification of PPARγ does not alter its adipogenic capacity, but leads to dysregulation of a large number of genes whose expression is altered in obesity, including a reduction in the expression of the insulin-sensitizing adipokine, adiponectin. The phosphorylation of PPARγ by Cdk5 is blocked by anti-diabetic PPARγ ligands, such as rosiglitazone and MRL24. This inhibition works both in vivo and in vitro, and is completely independent of classical receptor transcriptional agonism. Similarly, inhibition of PPARγ phosphorylation in obese patients by rosiglitazone is very tightly associated with the anti-diabetic effects of this drug. All these findings strongly suggest that Cdk5-mediated phosphorylation of PPARγ may be involved in the pathogenesis of insulin-resistance, and present an opportunity for development of an improved generation of anti-diabetic drugs through PPARγ.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Kershaw, E. E. & Flier, J. S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 89, 2548–2556 (2004)
Hotamisligil, G. S., Shargill, N. S. & Spiegelman, B. M. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 259, 87–91 (1993)
Lagathu, C. et al. Long-term treatment with interleukin-1β induces insulin resistance in murine and human adipocytes. Diabetologia 49, 2162–2173 (2006)
Steppan, C. M. et al. The hormone resistin links obesity to diabetes. Nature 409, 307–312 (2001)
Berg, A. H. et al. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nature Med. 7, 947–953 (2001)
Hu, E., Liang, P. & Spiegelman, B. M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 271, 10697–10703 (1996)
Yamauchi, T. et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature Med. 7, 941–946 (2001)
Morrison, R. F. & Farmer, S. R. Hormonal signaling and transcriptional control of adipocyte differentiation. J. Nutr. 130, 3116S–3121S (2000)
Tontonoz, P., Hu, E. & Spiegelman, B. M. Stimulation of adipogenesis in fibroblasts by PPAR γ 2, a lipid-activated transcription factor. Cell 79, 1147–1156 (1994)
Willson, T. M., Lambert, M. H. & Kliewer, S. A. Peroxisome proliferator-activated receptor γ and metabolic disease. Annu. Rev. Biochem. 70, 341–367 (2001)
Jimenez, M. A. et al. Critical role for Ebf1 and Ebf2 in the adipogenic transcriptional cascade. Mol. Cell. Biol. 27, 743–757 (2007)
Wu, Z., Bucher, N. L. & Farmer, S. R. Induction of peroxisome proliferator-activated receptor γ during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPβ, C/EBPδ, and glucocorticoids. Mol. Cell. Biol. 16, 4128–4136 (1996)
Lehmann, J. M. et al. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPAR γ). J. Biol. Chem. 270, 12953–12956 (1995)
Trujillo, M. E. & Scherer, P. E. Adipose tissue-derived factors: impact on health and disease. Endocr. Rev. 27, 762–778 (2006)
Sharma, A. M. & Staels, B. Peroxisome proliferator-activated receptor γ and adipose tissue — understanding obesity-related changes in regulation of lipid and glucose metabolism. J. Clin. Endocrinol. Metab. 92, 386–395 (2007)
Lipscombe, L. L. et al. Thiazolidinediones and cardiovascular outcomes in older patients with diabetes. J. Am. Med. Assoc. 298, 2634–2643 (2007)
Willson, T. M. et al. The structure-activity relationship between peroxisome proliferator-activated receptor gamma agonism and the antihyperglycemic activity of thiazolidinediones. J. Med. Chem. 39, 665–668 (1996)
Acton, J. J., III et al. Benzoyl 2-methyl indoles as selective PPARγ modulators. Bioorg. Med. Chem. Lett. 15, 357–362 (2005)
Dhavan, R. & Tsai, L. H. A decade of CDK5. Nature Rev. Mol. Cell Biol. 2, 749–759 (2001)
Utreras, E. et al. Tumor necrosis factor-α regulates cyclin-dependent kinase 5 activity during pain signaling through transcriptional activation of p35. J. Biol. Chem. 284, 2275–2284 (2009)
Rosen, E. D. et al. C/EBPα induces adipogenesis through PPARγ: a unified pathway. Genes Dev. 16, 22–26 (2002)
Musa, F. R. et al. Effects of luteinizing hormone, follicle-stimulating hormone, and epidermal growth factor on expression and kinase activity of cyclin-dependent kinase 5 in Leydig TM3 and Sertoli TM4 cell lines. J. Androl. 21, 392–402 (2000)
Torti, F. M. et al. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science 229, 867–869 (1985)
Cinti, S. The adipose organ. Prostaglandins Leukot. Essent. Fatty Acids 73, 9–15 (2005)
Leesnitzer, L. M. et al. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry 41, 6640–6650 (2002)
Sarraf, P. et al. Loss-of-function mutations in PPARγ associated with human colon cancer. Mol. Cell 3, 799–804 (1999)
Berger, J. P. et al. Distinct properties and advantages of a novel peroxisome proliferator-activated protein γ selective modulator. Mol. Endocrinol. 17, 662–676 (2003)
Gregoire, F. M. et al. MBX-102/JNJ39659100, a novel peroxisome proliferator-activated receptor-ligand with weak transactivation activity retains antidiabetic properties in the absence of weight gain and edema. Mol. Endocrinol. 23, 975–988 (2009)
Ostberg, T. et al. A new class of peroxisome proliferator-activated receptor agonists with a novel binding epitope shows antidiabetic effects. J. Biol. Chem. 279, 41124–41130 (2004)
Hakak, Y. et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc. Natl Acad. Sci. USA 98, 4746–4751 (2001)
Salim, C. et al. The giant protein AHNAK involved in morphogenesis and laminin substrate adhesion of myelinating Schwann cells. Glia 57, 535–549 (2009)
Merino-Trigo, A. et al. Sorting nexin 5 is localized to a subdomain of the early endosomes and is recruited to the plasma membrane following EGF stimulation. J. Cell Sci. 117, 6413–6424 (2004)
Maier, C. S. & Deinzer, M. L. Protein conformations, interactions, and H/D exchange. Methods Enzymol. 402, 312–360 (2005)
Nolte, R. T. et al. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-γ. Nature 395, 137–143 (1998)
Bruning, J. B. et al. Partial agonists activate PPARγ using a helix 12 independent mechanism. Structure 15, 1258–1271 (2007)
Flier, J. S. et al. Severely impaired adipsin expression in genetic and acquired obesity. Science 237, 405–408 (1987)
Calle, E. E. et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 348, 1625–1638 (2003)
Whitmer, R. A. et al. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. Br. Med. J. 330, 1360–1362 (2005)
Patrick, G. N. et al. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 402, 615–622 (1999)
Kinsella, T. M. & Nolan, G. P. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum. Gene Ther. 7, 1405–1413 (1996)
Walkey, C. J. & Spiegelman, B. M. A functional peroxisome proliferator-activated receptor-γ ligand-binding domain is not required for adipogenesis. J. Biol. Chem. 283, 24290–24294 (2008)
Lockhart, D. J. et al. Expression monitoring by hybridization to high-density oligonucleotide arrays. Nature Biotechnol. 14, 1675–1680 (1996)
Li, C. & Wong, W. H. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc. Natl Acad. Sci. USA 98, 31–36 (2001)
Moreno-Navarrete, J. M. et al. Complement factor H is expressed in adipose tissue in association with insulin resistance. Diabetes 59, 200–209 (2010)
Kloting, N. et al. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab. 6, 79–87 (2007)
Bluher, M. et al. Relation between glycaemic control, hyperinsulinaemia and plasma concentrations of soluble adhesion molecules in patients with impaired glucose tolerance or Type II diabetes. Diabetologia 45, 210–216 (2002)
Acknowledgements
We thank V. Mootha for help with the analysis of microarray data and for critical comments. We thank M. Kirschner for a critical reading of the manuscript and for comments. We are grateful to R. Gupta and P. Cohen for critical comments on the manuscript. J.H.C., A.S.B., J.L.E., P.B., D.L., J.L.R. and B.M.S are supported by NIH DK31405. S.K. is supported by NIH grant DK087853. M.B. is supported by a grant from Deutsche Forschungsgemeinschaft (DFG) and the Clinical Research group ‘Atherobesity’ KFO 152 (project BL 833/1-1). P.R.G., M.J.C. and T.M.K. are supported in part by the by the Intramural Research Program of the N National Institute of Mental Health (P.R.G., M.J.C., T.M.K., U54-MH084512; H. Rosen Principal Investigator) and by the NIH National Institute of General Medical Sciences (P.R.G. and M.J.C., R01-GM084041).
Author information
Authors and Affiliations
Contributions
J.H.C. and B.M.S. conceived and designed the experiments. J.H.C., A.S.B., J.L.E., S.K., P.B., D.L., J.L.R., M.J.C., T.M.K, M.B. and P.R.G performed the experiments. All authors analysed the data. J.H.C., A.S.B., J.L.E. and B.M.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Microarray data have been deposited in Gene Expression Omnibus: GSE22033.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-16 with legends and Supplementary Tables 1-4. (PDF 1157 kb)
Rights and permissions
About this article
Cite this article
Choi, J., Banks, A., Estall, J. et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARγ by Cdk5. Nature 466, 451–456 (2010). https://doi.org/10.1038/nature09291
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature09291
This article is cited by
-
Whole transcriptome mapping reveals the lncRNA regulatory network of TFP5 treatment in diabetic nephropathy
Genes & Genomics (2024)
-
Effects of half-dose spiomet treatment in girls with early puberty and accelerated bone maturation: a multicenter, randomized, placebo-controlled study protocol
Trials (2023)
-
3-Hydroxybutyrate ameliorates insulin resistance by inhibiting PPARγ Ser273 phosphorylation in type 2 diabetic mice
Signal Transduction and Targeted Therapy (2023)
-
Blockage of PPARγ T166 phosphorylation enhances the inducibility of beige adipocytes and improves metabolic dysfunctions
Cell Death & Differentiation (2023)
-
Lysine 222 in PPAR γ1 functions as the key site of MuRF2-mediated ubiquitination modification
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.