Abstract
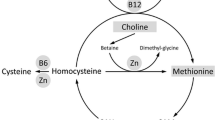
India is world’s capital for low birth weight (LBW), which is ascribed to intrauterine growth restriction (IUGR) rather than prematurity. An average Indian mother is short and thin and gives birth to a light and thin baby. Maternal undernutrition is thought to be a major factor in the aetiology of IUGR, and the undernutrition is usually thought to be a low macronutrient intake. The Pune Maternal Nutrition Study (PMNS) showed that the Indian babies were thin but fat (more adipose) compared to European babies, and that maternal intake of micronutrient-rich foods was a strong determinant of fetal size. Two thirds of the mothers had low vitamin B12 concentrations, folate deficiency was rare, and high circulating concentrations of homocysteine predicted IUGR. Follow up of these children revealed that higher maternal folate in pregnancy predicted higher adiposity and insulin resistance at 6 years of age. The most insulin resistant children were born to mothers who were vitamin B12 deficient and had high folate concentrations. Thus, PMNS suggests an important role for maternal one-carbon (1C) metabolism in fetal growth and programming of diabetes risk. This could be due to the role of 1C metabolism in synthesis of nucleic acids, genomic stability and the epigenetic regulation of gene function. In addition, methionine has important role in protein synthesis. These ideas are supported by animal studies. The next logical step in India will be to improve 1C metabolism in adolescents to effect intergenerational prevention of adiposity, diabetes and other related conditions.



Similar content being viewed by others
References
United Nations Children’s Fund and World Health Organization. Low birthweight: country, regional and global estimates. New Yor: UNICEF; 2004.
Hales CN, Barker DJP. Type 2 (non-insulin dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601.
Barker DJP. Fetal nutrition and cardiovascular disease in later life. Br Med Bul. 1997;53:96–108.
Harding JE. The nutritional basis of the fetal origins of adult disease. Int J Epidemiol. 2001;30:15–23.
Davey Smith G, Ebrahim S, Lewis S, Hansell AL, Palmer LJ, Burton PR. Genetic epidemiology and public health: hope, hype, and future prospects. Lancet. 2005;366:1484–98.
Freathy RM, Mook-Kanamori DO, Sovio U, Prokopenko I, Timpson NJ, Berry DJ, et al. Variants in ADCY5 and near CCNL1 are associated with fetal growth and birth weight. Nat Genet. 2010;42:430–5.
McCance RA. Food, growth, and time. Lancet. 1962;2:621–6.
Walton A, Hammond J. The maternal effects on growth and conformation in shire horse-shetland pony crosses. Proc Roy Soc Lond B Biol Sci. 1938;840:311–35.
Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum Dev. 2006;82:485–91.
Pedersen J: Hyperglycaemia-hyperinsulinism theory and birthweight. In: The pregnant diabetic and her new born: problems and management. Williams & Wilkins, Baltimore, 1977, pp 211–20
Freinkel N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes. 1980;29:1023–35.
Pettitt DJ, Aleck KA, Baird HR, Carraher MJ, Bennett PH, Knowler WC. Congenital susceptibility to NIDDM: role of intrauterine environment. Diabetes. 1988;37:622–8.
Forrester T. Historic and early life origins of hypertension in Africans. J Nutr. 2004;134:211–6.
Adair LS, Prentice AM. A critical evaluation of the fetal origins hypothesis and its implications for developing countries. J Nutr. 2004;134:191–3.
Yajnik CS. Early life origins of insulin resistance and type 2 diabetes in India and other Asian countries. J Nutr. 2004;134:205–10.
Li Y, He Y, Qi L, Jaddoe VW, Feskens EJ, Yang X, et al. Exposure to the Chinese famine in early life and the risk of hyperglycemia and type 2 diabetes in adulthood. Diabetes. 2010;59:2400–6.
Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300:2886–97.
Lucas A. Programming by early nutrition in man. In: Bock GR, Whelan J, editors. The childhood environment and adult disease. CIBA Foundation Symposium 1991. 156. Wiley. Chichester pp 38–55
Yajnik CS, Fall CHD, Vaidya U, Pandit AN, Bavdekar A, Bhat DS, et al. Fetal growth and glucose and insulin metabolism in four year old Indian children. Diabet Med. 1995;12:330–6.
Bavdekar A, Yajnik CS, Fall CH, Bapat S, Pandit AN, Deshpande V, et al. Insulin resistance syndrome in 8-year-old Indian children: small at birth, big at 8 years, or both? Diabetes. 1999;48:2422–9.
Rao S, Yajnik CS, Kanade A, Fall CH, Margetts BM, Jackson AA, et al. Intake of micronutrient-rich foods in rural Indian mothers is associated with the size of their babies at birth: Pune Maternal Nutrition Study. J Nutr. 2001;131:1217–24.
Fall CHD, Yajnik CS, Rao S, Coyaji KJ. The effects of maternal body composition before pregnancy on fetal growth; The Pune Maternal Nutrition Study. In: Shaughn O’Brien PM, Wheeler T, Barker DJP, editors. Fetal programming influences on development and disease in later life, chapter 21. London: RCOG; 1999. p. 231–45.
Joshi NP, Kulkarni SR, Yajnik CS, Joglekar CV, Rao S, Coyaji KJ, et al. Increasing maternal parity predicts neonatal adiposity: Pune Maternal Nutrition Study. Am J Obstet Gynecol. 2005;193:783–9.
Kinare AS, Natekar AS, Chinchwadkar MC, Yajnik CS, Coyaji KJ, Fall CH, et al. Low midpregnancy placental volume in rural Indian women: A cause for low birth weight? Am J Obstet Gynecol. 2000;182:443–8.
Yajnik CS, Fall CHD, Coyaji KJ, Hirve SS, Rao S, Barker DJ, et al. Neonatal anthropometry: the thin-fat Indian baby: The Pune Maternal Nutrition Study. Int J Obes. 2003;26:173–80.
Selhub J. Public health significance of elevated homocysteine. Food Nutr Bull. 2008;29:S116–25.
Yajnik CS, Deshpande SS, Jackson AA, Refsum H, Rao S, Fisher D, et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: The Pune Maternal Nutrition Study. Diabetologia. 2008;51:29–38.
Yajnik CS, Deshpande SS, Panchanadikar AV, Naik SS, Deshpande JA, Coyaji KJ, et al. Maternal total homocysteine concentration and neonatal size in India. Asia Pac J Clin Nutr. 2005;14:179–81.
Muthayya S, Kurpad AV, Duggan CP, Bosch RJ, Dwarkanath P, Mhaskar A, et al. Low maternal vitamin B12 status is associated with intrauterine growth retardation in urban South Indians. Eur J Clin Nutr. 2006;60:791–801.
Bhate V, Deshpande S, Bhat D, Joshi N, Ladkat R, Watve S, et al. Vitamin B12 status of pregnant Indian women and cognitive function in their 9-year-old children. Food Nutr Bull. 2008;29:249–54.
Godbole K, Gayathri P, Ghule S, Sasirekha BV, Kanitkar-Damle A, Memane N, et al. Maternal one-carbon metabolism, MTHFR and TCN2 genotypes and neural tube defects in India. Birth Defects Res A Clin Mol Teratol. 2011. doi:10.1002/bdra.20841.
Krishnaveni GV, Hill JC, Veena SR, Bhat DS, Wills AK, Karat CL, et al. Low plasma vitamin B (12) in pregnancy is associated with gestational “diabesity” and later diabetes. Diabetologia. 2009;52:2350–8.
Mook-Kanamori DO, Marsh JA, Warrington NM, Taal HR, Newnham JP, Beilin LJ, et al. Variants near CCNL1/LEKR1 and in ADCY5 and fetal growth characteristics in different trimesters. J Clin Endocrinol Metab. 2011;96:E810–5.
Hattersley AT, Beards F, Ballantyne E, Appleton M, Harvey R, Ellard S. Mutations in the glucokinase gene of the fetus result in reduced birth weight. Nat Genet. 1998;19:268–70.
Van Speybroeck L. From epigenesis to epigenetics: the case of C.H. Waddington. Ann N Y Acad Sci. 2002;981:61–81.
Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD, Hanson MA. Epigenetic mechanisms and the mismatch concept of the developmental origins of health and disease. Pediatr Res. 2007;61:5R–10R.
Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–300.
Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135:1382–6.
Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, Bispham J, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci USA. 2007;104:19351–6.
Yajnik CS, Deshpande SS, Lubree HG, Naik SS, Bhat DS, Uradey BS, et al. Vitamin B12 deficiency and hyperhomocysteinemia in rural and urban indians. J Assoc Phys India. 2006;54:1–8.
Yajnik CS. Nutrient-mediated teratogenesis and fuel-mediated teratogenesis: two pathways of intrauterine programming of diabetes. Int J Gynaecol Obstet. 2009;104:S27–31.
International Diabetes Federation. Diabetes Atlas. International Diabetes Federation, Brussels, Belgium (2009) www.diabetesatlas.org/ (Accessed on 12th September 2011)
Acknowledgement
We are funded by the Wellcome Trust (London, UK); the Nestlé Foundation (Lausanne, Switzerland); The International Atomic Energy Agency (Vienna, Austria); The Department of Biotechnology (DBT), Government of India (New Delhi, India); and Sight and Life, Basel, Switzerland.
Thanks are due to colleagues, collaborators, field workers, and parents and children who participated in the studies mentioned in this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yajnik, C.S., Deshmukh, U.S. Fetal programming: Maternal nutrition and role of one-carbon metabolism. Rev Endocr Metab Disord 13, 121–127 (2012). https://doi.org/10.1007/s11154-012-9214-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-012-9214-8