Abstract
Objective
Antibiotics are the drugs most frequently prescribed for children, and most of them lack patent protection. The aim of this study was to evaluate off-label antibiotic use in three European countries.
Methods
Data relating to all patients admitted to the neonatal intensive care units (NICUs) and paediatric wards of the participating centres were collected by the same investigator over a 2-week survey period between February and May 2009. The data included age, date of birth, weight, relevant medical history and diagnosis, together with details of all of the antibiotics prescribed (compound, route of administration, dose, and indication for use).
Results
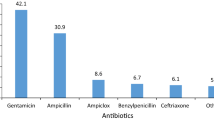
The study involved 616 children (110 admitted to NICUs: 62 in the UK, 38 in Italy and 10 in Greece; 506 admitted to general paediatric wards: 265 in the UK, 94 in Italy and 147 in Greece). A total of 1244 antibiotic prescriptions were issued (290 in NICUs and 954 in paediatric wards). The results showed that off-label antibiotic use is very common among European paediatric patients, with generally only slight, but sometimes significant differences between countries. However, this use relates almost exclusively to doses and indications, and rarely to age. The only antibiotics found to be used off-label in an age-related manner in paediatric clinical practice are meropenem for neonates and quinolones or linezolid for older children, which represent priorities for future studies.
Conclusion
European-wide educational programmes are urgently needed to meet the objectives of improving paediatricians’ working knowledge of the recommendations surrounding licensed antibiotics-use in children, and of reducing uncontrolled patterns of prescribing.
Similar content being viewed by others
References
Conroy S, McIntre J, Choonora I (1999) Unlicensed and off label drug use in neonates. Arch Dis Child Fetal Neonatal Ed 80:F142–F144
Conroy S, Choonara I, Impicciatore I et al (2000) Survey of unlicensed and off label drug use in paediatric wards in European countries. European Network for Drug Investigation in Children. Br Med J 320:79–82
Turner S, Longworth A, Nunn AJ, Choonara I (1998) Unlicensed and off label drug use in paediatric wards: prospective study. Br Med J 316:343–345
‘t Jong GW, Vulto AG, de Hoog M, Schimmel KJ, Tibboel D, van den Anker JN (2001) A survey of the use of off-label and unlicensed drugs in Dutch children’s hospital. Pediatrics 108:1089–1093
Schirm E, Tobi H, Jong-Van D, den Berg LTW (2002) Unlicensed and off label drug use by children in the community: cross sectional study. Br Med J 324:1312–1313
Bucheler R, Schwab M, Morike K et al (2002) Off-label prescribing to children in primary care in Germany: retrospective cohort study. Br Med J 324:1311–1312
Pandolfini C, Campi R, Clavenna A, Cazzato T, Bonati M (2005) Italian paediatricians and off-label prescriptions: loyal to regulatory or guideline standards? Acta Paediatr 94:753–757
Sammons H, Conroy S (2008) How do we ensure safe prescribing for children? Arch Dis Child 93:98–99
Conroy S, Carroll WD (2009) Prescribing in pediatrics. Arch Dis Child Educ Pract Ed 94:55–59
Tafuri G, Trotta F, Leufkens HGM, Martini N, Sagliocca L, Traversa G (2009) Off-label use of medicines in children: can available evidence avoid useless pediatric trials? Eur J Clin Pharmacol 65:209–216
de Jong J, van den Berg PB, Visser ST, de Vries TW, de Jong-van den Berg LTW (2009) Antibiotic usage, dosage and course length in children between 0 and 4 years. Acta Paediatr 98:1142–1148
Lenk C, Koch P, Zappel H, Wiesemann C (2009) Off-label, off-limits? Parental awareness and attitudes towards off-label use in paediatrics. Eur J Pediatr 168:1473–1478
Conroy S, McIntyre J, Choonara I, Stephenson T (2000) Drug trials in children: problems and the way forward. Br J Clin Pharmacol 49:93–97
Pandolfini C, Bonati M, Sammons H (2009) Registration of trials in children. Update of current international initiatives Arch Dis Child 94:717–719
Altavilla A, Giaquinto C, Ceci A (2008) European survey on ethical and legal frame work of clinical trials in paediatrics: results and perspectives. J Int Bioethique 19:17–48
Holt D, Harvey D, Hurley R (1993) Chlorampenicol toxicity. Adverse Drug React Toxicol Rev 12:83–95
McIntyre J, Choonara I (2004) Drug toxicity in the neonate. Biol Neonate 86:218–221
Horen B, Montastruc JL, Lapeyre-Mestre M (2002) Adverse drug reactions and off-label drug use in paediatric outpatients. Br J Clin Pharmacol 54:665–670
Clarkson A, Choonara I (2002) Surveillance for fatal suspected adverse drug reactions in the UK. Arch Dis Child 87:462–466
Choonara I, Conroy S (2002) Unlicensed and off-label drug use in children: implications for safety. Drug Saf 25:1–5
Turner S, Nunn AJ, Fielding K, Choonara I (1999) Adverse drug reactions to unlicensed and off-label drugs on paediatric wards: a prospective study. Acta Paediatr 88:965–968
Johann-Liang R, Wyeth J, Chen M, Cope JU (2009) Pediatric drug surveillance and the Food and Drug Administration’s adverse event reporting system: an overview of reports, 2003–2007. Pharmacoepidemiol Drug Saf 18:24–27
Best Pharmaceuticals for Children Act. Pub. L. 107–109, 107th Cong, 2002
Pediatric Research Equity Act. Pub. L. No. 108155 (S.650), 2003
Neubert A, Wong IC, Bonifazi A et al (2008) Defining off-label and unlicensed use of medicines for children: results of a Delphi survey. Pharmacol Res 58:316–322
Ceci A, Felisi M, Baiardi P et al (2006) Medicines for children licensed by the European Medicines Agency (EMEA): the balance after 10 years. Eur J Clin Pharmacol 62:947–952
Marchetti F, Bua J, Ventura A, Notarangelo LD, Di Maio S, Migliore G, Bonati M (2007) The awareness among paediatricians of off-label prescribing in children: a survey of Italian hospitals. Eur J Clin Pharmacol 63:81–85
Clavenna A, Sequi M, Bortolotti A, Merlino L, Fortino I, Bonati M (2009) Determinants of the drug utilization profile in the paediatric population in Italy’s Lombardy Region. Br J Clin Pharmacol 67:565–571
Burke C (2009) Perinatal sepsis. J Perinat Neonatal Nurs 23:42–51
Fernando AM, Heath PT, Menson EN (2008) Antimicrobial policies in the neonatal units of the United Kingdom and Republic of Ireland. J Antimicrob Chemother 61:743–745
Arrieta A (1997) Use of meropenem in the treatment of serious infections in children: review of the current literature. Clin Infect Dis 24[Suppl 2]:S207–S212
Principi N, Marchisio P (1998) Meropenem compared with ceftazidime in the empiric treatment of acute severe infections in hospitalized children. J Chemother 10:108–113
Mandell LA, Peterson LR, Wise R et al (2002) The battle against emerging antibiotic resistance: should fluoroquinolones be used to treat children? Clin Infect Dis 35:721–727
Schaad UB (2005) Fluoroquinolone antibiotics in infants and children. Infect Dis Clin North Am 19:617–628
Velissarious IM (2006) Use of linezolid in children: an overiew of recent advances. Expert Rev Anti Infect Ther 4:947–952
Kosaka T, Kokufu T, Shime N, Sugioka N, Kato R, Hamaoka K, Fujita N (2009) Pharmacokinetics and tolerance of linezolid for meticillin-resistant Staphylococcus aureus mediastinitis in paediatric patients. Int J Antimicrob Agents 33:368–370
Santos RP, Prestidge CB, Brown ME et al (2009) Pharmacokinetics and pharmacodynamics of linezolid in children with cystic fibrosis. Pediatr Pulmonol 44:148–154
Rao SC, Ahmed M, Hagan R (2006) One dose per day compared to multiple doses per day of gentamicin for treatment of suspected or proven sepsis in neonates. Cochrane Database Syst Rev 1:CD005091
Hagen I, Øymar K (2009) Pharmacological differences between once daily and twice daily gentamicin dosage in newborns with suspected sepsis. Pharm World Sci 31:18–23
Dowell SF, Butler JC, Giebink GS et al (1999) Acute otitis media: management and surveillance in an era of pneumococcal resistance—a report from the Drug-resistant Streptococcus pneumoniae Therapeutic Working group. Pediatr Infect Dis J 18:1–9
Piglansky L, Leibovitz E, Raiz S, Greenberg D, Press J, Leiberman A, Dagan R (2003) Bacteriologic and clinical efficacy of high dose amoxicillin for therapy of acute otitis media in children. Pediatr Infect Dis J 22:405–413
Principi N, Esposito S, Blasi F, Allegra L, the Mowgli Study Group (2001) Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community-acquired lower respiratory tract infections. Clin Infect Dis 32:1281–1289
Esposito S, Blasi F, Allegra L, Principi N, the Mowgli Study Group (2001) The use of antimicrobials for community-acquired lower respiratory tract infections in hospitalized children. Eur J Clin Microbiol Infect Dis 20:647–650
Principi N, Esposito S (2001) Emerging role of Mycoplasma pneumoniae and Chlamydia pneumoniae in paediatric respiratory tract infections. Lancet Infect Dis 1:334–344
Acknowledgements
We would like to thank all of our colleagues who contributed to the data collection: Fabio Mosca, Lorenza Pugni, Maria Cristina Pietrogrande, Rosa Maria Dellepiane, Edoardo Calderini and Maurizio Torricelli at the Department of Maternal and Pediatric Sciences, Università degli Studi di Milano, Fondazione IRCCS Ospedale Maggiore Policlinico, Mangiagalli e Regina Elena, Milan, Italy; Dimitris Kafetzis, George Kourakis, George Petousis and Alexandros Passalidis at P. and A. Kyriakou Children’s Hospital, University of Athens, Athens, Greece.
The authors have no conflict of interest to declare. This study was supported in part by a grant from the Pediatric Unit 1, Fondazione IRCCS Ospedale Maggiore Policlinico, Mangiagalli e Regina Elena, Milan, Italy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Porta, A., Esposito, S., Menson, E. et al. Off-label antibiotic use in children in three European countries. Eur J Clin Pharmacol 66, 919–927 (2010). https://doi.org/10.1007/s00228-010-0842-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-010-0842-1