Article Text
Abstract
Objectives Our meta-analysis performed a systematic evaluation on the therapeutic efficacy and safety of tumour vaccines for the treatment of advanced non-small cell lung cancer (NSCLC).
Design Systematic review and meta-analysis of randomised controlled trials (RCT).
Data sources PubMed, the Cochrane Center Register of Controlled Trials, Science Direct and EMBASE were searched from January 1980 until January 2015.
Eligibility criteria for selecting studies RCT were included; the control arm had to receive either placebo or chemotherapy or no treatment.
Main outcome measures The quality of the data from individual papers was assessed for overall survival (OS), clinical response rate and side effects.
Results Overall, 11 RCT of advanced NSCLC with a total of 3986 patients were conducted for meta-analysis. The results showed that the vaccine arm significantly extended primary endpoint median overall survival compared with control group (p<0.00001) (HR 0.760; 95% CI 0.644 to 0.896; p=0.001). Three subgroup patients with tumour vaccine at 1-year, 2-year and 3-year survival rates also gained significant benefits compared with their corresponding control group (p=0.0004, 0.03 and 0.19, respectively). Besides, a significant improvement in median time to progression (TTP), median progression-free survival (PFS) and a trend of improvement in objective response rate were observed after tumour vaccine treatment (p=0.001, 0.005 and 0.05, respectively; median PFS HR 0.842; 95% CI 0.744 to 0.954; p=0.007). A few severe adverse effects occurred in the tumour vaccine group, but fewer side effects were observed in the vaccine group compared with the control group (p<0.00001).
Conclusions Taken together, NSCLC tumour vaccines markedly prolong median OS (p<0.00001), median TTP (p=0.001) and median PFS (p=0.005), improve clinical response rate (p=0.05) and lessen adverse side effects (p<0.00001). Our meta-analysis suggests tumour vaccines improve the efficacy of the treatment, and also provide superiority in treatment of patients with advanced NSCLC among a variety of immunotherapy strategies.
- Tumor vaccine
- Immunotherapy
- Non-small cell lung cancer
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Strengths and limitations of this study
The methodological quality of each involved paper was evaluated with the Jadad Scale and publication bias analysed.
Eleven selected studies involving seven different tumour vaccines were included, which may influence reliability of assessment of efficiency due to insufficient clinical outcomes of other non-small cell lung cancer tumour vaccines.
All selected studies were randomised controlled trials, but some were open label, which may lead to distribution and implementation bias in the present analysis.
Introduction
Lung cancer is the leading morbidity and mortality disease worldwide, with an estimated 13% cancer-related mortality attributed to the disease based on the 2012 Chinese Cancer Registration Annual Report.1 Non-small cell lung cancer (NSCLC) accounts for almost 85% of all lung cancer cases. A third-generation platinum-based doublets regimen results in a median overall survival (OS) of 10 months and a 1-year survival rate of approximately 40% for patients with unresectable locally advanced or metastatic disease.2 Novel treatment strategies need to be explored for improving clinical outcomes. A recently developed therapeutic cancer vaccine has turned out to be a promising strategy for advanced NSCLC (stages III–IV). The major advantage of vaccination is that it can generate a strong and long-lasting response to antigens. Cancer vaccination relies on specific priming of the immune system to stimulate innate immunity by identification of relevant target antigens coupled with a sophisticated delivery adjuvant.3
Several approaches of immunotherapy have been proposed. Active immunotherapy aims at inducing an endogenous immune response after administration of vaccines. Passive immunotherapy transfers an ex vivo expansion of the immunological effector cells into the host or tumour targeted antibodies.4 Others are targeted at potentiating the immune response via cytokines (interleukin (IL) 2, interferons (IFNs), etc) or molecular target agents.5 The vaccine formulation is based on immunogenic tumour-associated antigens and adjuvants. The antigen is made up of specific peptides, recombinant proteins and whole tumour lysates or irradiated tumour cells. The immunoadjuvant is used to potentiate the immune response and consists of phospholipid, aluminium formulation, viral vector, dendritic cell or liposome.6
Patients with NSCLC were initially not considered suitable candidates for vaccine therapy treatment due to the weak immunogenicity of NSCLC.3 To date, several solutions have been proposed. Efficient lung tumour-specific antigens have been identified and presented in the optimal immunoadjuvants in the vaccine to induce a therapeutic response. A large number of clinical trials as well as studies indicated that the following classifications of therapeutic cancer vaccines present promising clinical outcomes: full protein vaccines (MAGE-A3 vaccine, CimaVax EGF vaccine), viral vector vaccine (TG4010 vaccine), peptide vaccine (L-BLP25 vaccine), whole tumour cell vaccine (GVAX vaccine), ganglioside vaccine (Racotumomab-Alum vaccine) and Belagenpumatucel vaccine (Lucanix vaccine).7–9
According to the results of phase II–III clinical trials, the therapeutic cancer vaccines have been evaluated to be effective and safe therapies for advanced NSCLC among various strategies of immunotherapies. There are a few potential therapeutic cancer vaccines undergoing phase III clinical studies, such as Lucanix (Belagenpumatucel, NCT00676507), TIME (TG4010, NCT01383148), INSPIRE (L-BLP25, NCT01015443), MAGRIT (MAGE-A3, NCT00480025) and CimaVax (EGF, NCT01444118).8 ,9 Even though these potential therapeutic cancer vaccines have been documented in many studies, there is no systematic review to assess the therapeutic efficacy of cancer vaccines combined with chemotherapy in NSCLC. Therefore, we performed a systematic meta-analysis of cancer vaccines with randomised controlled trials (RCT) on NSCLC clinical phases II–III. In this large-scale study, we evaluated the clinical efficiency and safety of the therapeutic cancer vaccines on patients with advanced NSCLC.
Methods
Study design, search strategy and eligibility criteria
The relevant trials in this study were identified by searching PubMed, the Cochrane Center Register of Controlled Trials, ScienceDirect and EMBASE, for RCT from January 1980 until January 2015. The search strategy included the keywords ‘non-small-cell lung cancer’, ‘tumor vaccine’, ‘immunotherapy’ and free text search. In addition, we manually searched a website of clinical trials for ongoing trials. We searched with keywords ‘non-small-cell lung cancer’, and ‘tumor vaccine’ on website http://www.clinicaltrials.gov/. The registered clinical trials with publication citations are displayed at the bottom of the ‘Full Text View’ tab of a study record. Furthermore, we performed manual searches in the latest abstract of the American Society of Clinical Oncology (ASCO) Annual Meetings World Conference on Lung Cancer (WCLC) and the European Cancer Conference (ECCO). Reference lists of published trials and relevant review articles were also examined for published clinical results. No language restriction was applied.
Data selection criteria
Data extraction was independently conducted by two reviewers (MW and J-XC) using a standardised approach. Disagreement was adjudicated by a third reviewer (Z-XW) after referring back to the original publications. The selection criteria were: (1) English language studies were limited to human clinical trials; (2) RCT with tumour vaccine immunotherapy compared with control therapy for the treatment of late stage of NSCLC (III–IV) were included; (3) case studies, review articles and studies involving fewer than 10 patients were excluded and (4) phase I open label vaccine immunotherapy trials without control arm were excluded.
Patients and eligibility criteria
The main criteria for patient inclusion in the trials were: (1) male or female patients aged 18 years or older with histologically confirmed metastatic or locally advanced NSCLC (III-IV); (2) Eastern Collaborative Oncology Group (ECOG) performance status 0 or 1 and life expectancy of at least 4 months; (3) written informed consent from each patient in every study. The selective data are authors’ names, year of publication, sample size per arm, regimen used, tumour stage, median or mean age of patients, vaccine formulation, information pertaining to study design and main results of clinical efficacy in each arm.
Study quality assessment
The overall quality of each involved paper was evaluated by the Jadad Scale, which assessed the methodological quality of clinical trials before inclusion in a meta-analysis.10 A few of the major criteria were employed as a grading scheme: (1) randomisation; (2) allocation concealment; (3) blinding; (4) lost to follow-up; (5) intention to treat (ITT) and (6) baseline. Each criterion was graded as follows: A: adequate, with correct procedure; B: not described in sufficient detail to allow a definite judgment and C: inadequate procedures, methods, or information. The grades of each criterion were added up and used to compare study quality in a quantitative manner. Each involved study has been graded in the last column (quality grading) as follows: A: studies have a low risk of bias, which were scored as grade A for all items; B: studies have a moderate risk of bias with one or more grades of B; and C: studies have a high risk of bias with one or more grades of C. A funnel plot was used to evaluate the publication bias.11
Definition of outcome measures
The primary clinical end points in RCT for cancer therapies employed the measures of OS. The secondary end points were indicators of progression-free survival (PFS), time to progression (TTP) and the efficacy analyses-objective response rate (ORR) (ORR=complete response rates+partial rates). The side effects and toxicity were graded according to the National Cancer Institute Common Toxicity Criteria. The data were either obtained directly from the articles or calculated using the graphed data in articles using Photoshop and a software graph digitiser scout.
Statistical analysis
The analysis was performed by Review Manager V.5.0 (Nordic Cochran Centre, Copenhagen, Denmark) and STATA V.12.0 (StataCorp). A 1-year, 2-year and 3-year OS, ORR and side effects are reflected by OR, which was calculated by using a method reported by Mantel and Haenszel.12 For the analysis of clinical outcomes regarding median OS, median PFS and median TTP, mean difference was analysed by Revman V.5.0, along with the HR added in the results (STATA 12.0). In consideration of the possibility of heterogeneity among the studies, a statistical test for heterogeneity was examined by the χ2-based Q-test, and the significance level was fixed at p<0.10. The inconsistency index I2 was also calculated to evaluate the variation caused by heterogeneity. A high value of I2 indicated a higher probability of the existence of heterogeneity (p<0.10; I2>50%). When heterogeneity was confirmed, the random effects method was used. We pooled the effect estimates from the individual studies using a random effects model, which considered both within-study and between-study variations, yielding more conservative results than the fixed-effect model.13 A p value <0.05 was considered to be statistically significant. All reported p values were two sided.
Results
Selection of the trials
The data searches yielded a total of 120 references. After referring to the full texts, 79 were considered ineligible for different reasons (5 multiple cancer analyses, 26 reviews, 10 case reports, 23 in vitro experiments and 15 animal models). The remaining 41 articles were further evaluated, and 30 trials were excluded due to language, lack of RCT, different type of lung cancer and insufficient data. As a result, the final 11 articles were included for the meta-analysis. All of the selected studies were RCT and phase II–III clinical trials of tumour vaccine therapy treatment of NSCLC. The screening procedure is shown in figure 1.
Flow diagram of the study selection process.
The study quality was assessed using the Jadad Scale, generating grades ranging from A to C, with higher grade indicating better methodology. The quality assessment of the 11 studies is summarised in table 1.14–24 Three included trials showed Jadad scale A,14 ,15 ,19 and eight were B.16–18 ,20–24 The funnel plots for the seven analyses regarding 1-year, 2-year, and 3-year survival rates, ORR, median PFS, median TTP, median OS and side effects, were largely symmetrical. Thus, possible clinically important publication bias did not seem to be present in our meta-analysis.
Jadad Scale for the 11 randomised controlled studies
Characteristics of tumour vaccine therapy
The characteristics of the 11 trials are listed in table 2.14–24 Our selected 11 RCT, multicentre trials with 3986 patients with NSCLC in stages III–IV were included in the present analysis. The enrolled ages were between 30 and 89 years, with a median age greater than 55 years. A total of 10 studies were fully published text, and one abstract was included in our analysis as an important clinical outcome supplement.19 These studies are listed in table 2 and other related information is also listed.
Clinical information of patients from the eligible trials in the meta-analysis
The origins of patients’ information did not show any statistically significant difference between vaccine group and control group in all of our selected studies, with all p values >0.05. In our 11 selected trials, a number of 5 RCT trials did not apply chemotherapy as control arm; either best supportive care (BSC)16 ,17 ,24 or tumours harvested20 ,21 was performed as control arm where recruited patients all had stable disease or an objective clinical response after first-line treatment. The other six trials14 ,15 ,18 ,19 ,22 ,23 used chemotherapy as control group.
In all 11 studies, 3 major classifications of tumour vaccine were included: whole-cell vaccines,20 ,21 antigen-specific vaccines15–17 ,19 ,23 ,24 and non-antigen-specific immunomodulatory agents.14 ,18 ,22 Four of the selected studies evaluated tecemotide BLP25 liposome vaccine (L-BLP25, Stimuvax),15–17 ,19 a therapeutic cancer vaccine targeting the mucin 1 (MUC1) glycoprotein antigen that belongs to the antigen-specific vaccines. Two studies were clinical phase III trials, and patients were randomly assigned to either the tecemotide or placebo group.15 ,19 Two studies were phase IIB trials, and patients were randomised to either the L-BLP25 plus BSC or BSC group alone.16 ,17 One study observed the clinical outcome of therapeutic vaccination with TG4010,23 which consists of a suspension of a recombinant modified vaccinia virus strain Ankara (MVA), which codes for the MUC1 tumour-associated antigen and interleukin 2 (IL-2). One recombinant human recombinant epidermal growth factor (EGF) protein-specific therapeutic vaccine (CimaVax-EGF) was selected for analysis in our study.24 Other classification of whole-cell vaccines from two studies was collected for analysis of the clinical activity of GVAX vaccines, which are composed of autologous tumour cells genetically modified to secrete granulocyte–macrophage colony-stimulating factor (GM-CSF), in advanced-stage NSCLC.20 ,21 One study of phase III non-antigen-specific immunomodulatory agent-SRL172, which is a suspension of heat-killed Mycobacterium vaccae NCTC 11659, was analysed.22 Another immunomodulatory agent, the toll-like receptor 9 (TLR 9) agonist oligodeoxy nucleotide (PF-3512676),18 which is a synthetic nuclease-resistant, TLR9-activating oligodeoxy nucleotide that mimics the natural ligand of TLR9 (unmethylated CpG motifs), was collected in our study. Another promising vaccine, racotumomab, which consists of an monoclonal antibody (mAb) that mimics gangliosides with a glycosilation pattern almost exclusive of neoplastic cells, was employed in this analysis.14
Survival
The analysis results of OS are shown in figure 2. Three OS subgroups of the tumour vaccine group at 1-year, 2-year and 3-year survival rate, gained significant benefits compared with their corresponding control group (OR 1.52, 95% CI 1.25 to 1.84, p=0.0004; OR 1.41,95% CI 1.12 to 1.77, p=0.03; OR 1.36,95% CI 1.05 to 1.77, p=0.19, respectively) (figure 2). Seven trials with 2286 patients were selected in 1-year OS analysis, in which 1332 patients received vaccine treatment, while the other 954 patients were in the control group.14 ,15 ,18 ,20–22 The 1-year OS rates were 70% (935/1332) for patients who received therapeutic tumour vaccines, however, the control group only showed 58% (555/954) of 1-year OS rate. Without obvious heterogeneity, I2 revealed minor heterogeneity among individual studies; the 1-year OS also produced significant improvement compared with control group (p=0.0004, I2=31%). The relevant data on 2-year OS were available in three trials.14 ,15 ,17 A total of 1586 patients were included in 2-year OS analysis. The estimated pooled OR demonstrated that the vaccine group gained a significant improvement with 35% (355/1004) of 2-year survival rate versus 27% (157/582) for control group. There was moderate heterogeneity among individual studies on 2-year OS analysis (p=0.03, I2=48%). A total of two trials with 1410 patients was selected in 3-year OS analysis.15 ,16 The 3-year survival rate for the 917 patients receiving vaccine treatment was 27% (247/917), whereas a slightly lower survival rate was found for control group with 22.3% (110/493). The high heterogeneity presented in 3-year OS rate, and statistic difference, was not observed in 3-year OS rate (p=0.19, I2=80%). A random effects model was used for OS in analysis of three subgroups. As for median OS, the results of the pooled analysis showed that the vaccine arm significantly extended median OS at the end of follow-up compared with the control group, which was consistent with the OS (OR 1.44,95% CI 1.27 to 1.64, p<0.00001) (figure 2 and table 3).14–18 ,20 ,21 ,23 ,24
Comparison of M-OS, M-TTP and M-PFS, between the vaccine and control groups
Forest plot comparing the 1-year, 2-year and 3-year overall survival (OS) between the tumour vaccine group and control group. Owing to the low heterogeneity detected, the random effects model was used in this OS meta-analysis.
As an important primary clinical outcome, median OS was significantly prolonged in the vaccine group compared with control group (p<0.00001). Regarding median TTP and median PFS, the analysis results demonstrated that the tumour vaccine treatment group clearly extended in both clinical outcomes compared with the corresponding control group, which was consistent with median OS results (table 3) (p=0.001 and 0.005, respectively).14 ,17 ,19–23 With the heterogeneity observed across the studies, random effects models were used to analyse median TTP, median PFS and median OS. For the analysis of median OS and median PFS, the HR was additionally used as the common measure of association across studies. The summary median OS HR was 0.760 (95% CI 0.644 to 0.896; p=0.001), which demonstrated a statistically significant beneficial effect on the tumour vaccine treatment group. Little evidence of heterogeneity was found across the studies (I2=24.9%, p=0.001). The result of median PFS HR also showed consistency with median OS HR, with a summary HR of 0.842 (95% CI 0.744 to 0.954; p=0.007). Owing to insufficient data, the HR of median TTP was not available (table 3).
Furthermore, a high probability of heterogeneity existed in the analysis of median OS, median TTP and median PFS (table 3). To explore the potential source of heterogeneity across studies, a subgroup study was performed. Significant heterogeneity was still observed in the studies that conducted subgroup analysis by types of NSCLC tumour vaccine, control groups and the very large numbers of enrolled patients (I2>50%, p<0.05).
Response rate
The vaccine treatment group showed a favourable result when subjected to analysis of the ORR which was denoted by complete response rates (CR) and partial rates (PR), compared with the corresponding control arm (OR 1.36, 95% CI 1.02 to 1.80, p=0.05).17 ,18 ,22 ,23 Indeed, vaccine immunotherapy generated a statistical difference compared with the control arm in four trials including 849 patients. Within the random effects model used in ORR analysis, no evidence of heterogeneity among the individual studies was observed (I2=15%, p=0.05; figure 3).
Comparison of the objective response rate (ORR) between the tumour vaccine group and control group. The random effects model was used. Significant difference: p value <0.05.
Toxicity and adverse reactions
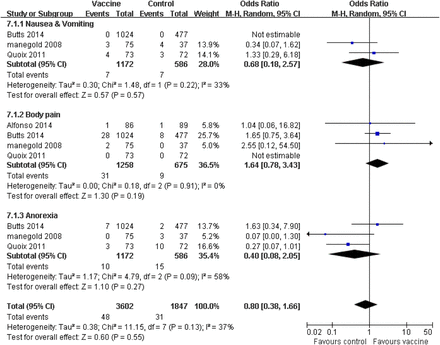
Treatment-related adverse effects (AEs) (any grades and grades ≥3) and efficacy for patients with advanced NSCLC were evaluated in the 11 collected studies. The patients in the vaccine group observed fewer obvious side effects compared with the corresponding control group, such as nausea and vomiting, thrombocytopaenia and injection site reaction (p≤0.05). Patients receiving tumour vaccine therapy experienced six other AEs, which occurred equally in the control group, including anaemia, leucopaenia, body pain, fatigue, fever, headache, anorexia, arthralgia and dyspnoea (p>0.05; figure 4). Furthermore, we separately considered the adverse effects of grades ≥3. The pooled analysis showed that the patients who received vaccine treatment experienced a few severe adverse effects (grades ≥3), including nausea and vomiting (p=0.57), body pain (p=0.19) and anorexia (p=0.27), which did not generate statistical difference compared with the corresponding control group (figure 5). Heterogeneity was observed; the random effects models were used for side effect and serious AE analysis.
Forest plot comparing the toxicity and treatment-related side effects between the tumour vaccine group and control group. The random effects meta-analysis model was used in this analysis.
Forest plot comparing the severe side effects (grades ≥3) between the tumour vaccine group and control group. Some serious adverse effects occurred equally in both groups. The random effects meta-analysis model was used in this analysis.
Discussion
NSCLC was initially thought to be a poorly immunogenic tumour, because a low number of tumour-infiltrating T cells were identified in the lesions.3 However, with the development of therapeutic tumour vaccines, specific NSCLC antigens and strong adjuvants were identified, which led to effective and safe clinical outcomes. A number of studies proved that patients with late-stage NSCLC who received tumour vaccine therapy combined with chemotherapy could gain a favourable prognosis compared with chemotherapy treatment alone.6 Our systemic review evaluated the efficacy and safety in the treatment of patients with NSCLC based on analysis of 1-year, 2-year and 3-year OS, median TTP, median PFS, median OS, clinical objective response and side effects.
Given the advanced stage in most patients, standard chemoradiotherapy has limited effect on clinical efficacy. An efficient alternative therapy is clearly needed. The results of the overall meta-analysis showed that the tumour vaccine group had a significant impact on 1-year, 2-year and 3-year OS compared with its corresponding control arm (figure 2) (p=0.0004, 0.03 and 0.19, respectively). According to our favourable results, the primary end point regarding median OS and second end points regarding median TTP and median PFS, showed favourable results in the tumour vaccine-treated group compared with the corresponding control group (p<0.00001, 0.001 and 0.005, respectively; table 3). In the present study, the tumour vaccine-treated group also indicated significant benefit in the clinical objective responses based on the assessment of traditional RECIST criteria.25 Collectively, our analysis demonstrates that tumour vaccine therapy may prove advantageous for patients with advanced NSCLC.
All the 11 enrolled studies were published by clinical phases II and III with random, multicentre trials in this meta-analysis, among which efficacy parameters were distributed to achieve better statistical reliability. Overall, most of the major criteria in the studies were achieved using the Jadad Scale, which indicated the high quality of the involved studies. Most of our collected studies have moderate risk of bias based on the assessment of the Jadad Scale. Because most of the enrolled studies were open label RCT instead of allocation concealment, double-blind RCT affected the quality assessment of the studies on the criteria of blinding and allocation concealment. To avoid bias in the identification and selection of trials, we collected as many NSCLC tumour vaccine treatment RCTs as possible to enlarge the data, and also minimised the possibility of overlooked publications based on our search strategy. Furthermore, a high probability of heterogeneity existed in the analysis of median OS, median TTP and median PFS (table 3). To explore the potential source of the heterogeneity across studies, a subgroup study was performed. Because there were differences in types of NSCLC tumour vaccines, types of control groups and specific number of enrolled patients within the included studies, which could modify the results of the included studies, we investigated the influence of these subgroups. The significant heterogeneity was still observed in the studies that conducted subgroup analysis by types of NSCLC tumour vaccines, control groups and the very large numbers of enrolled patients (I2>50%, p<0.05). Because of the limited number of NSCLC tumour vaccines evaluated, the source of heterogeneity across studies was not traceable. Other valuable ongoing trial results may provide more information.
Early attempts to develop NSCLC tumour vaccine had limited success due to failure to identify suitable target antigens, and failure to mitigate the immunosuppressive tumour environment and generate the immune escape.26 In the present meta-analysis, our results demonstrated that significant clinical efficacy and OS were achieved on the treatment of late-stage NSCLC. There are a few points that may explain this.
First, tumour vaccines improved tumour-specific antigen identification. Owing to the modification of tumour cell surface antigens, the weak immunogenicity protected the tumour cells from host immune destruction, which hardly induced tumour-specific immune response.3 However, NSCLC tumour-specific antigens have been identified to be attractive targets for tumour vaccines such as mucin 1 (MUC1), MAGE-A3, EGF antigens.27 MUC1 is a mucinous transmembrane glycoprotein that is over-expressed and under-glycosylated or aberrantly glycosylated in lung malignancies. High serum levels of MUC1 are associated with immune suppression and poor prognosis in patients with advanced adenocarcinoma.28 In our analysis, L-BLP25 vaccine and TG4010 vaccine, both targeting the MUC1 antigen, have been evaluated for clinical efficacy and have shown promising outcomes.15–17 ,19 ,23 Another antigen among the potential NSCLC vaccines, MAGE-A3, expressed in 35% of NSCLC but not in normal tissue, is considered a promising tumour vaccine.29 This tumour vaccine underwent the largest ever phase III lung cancer trial. However, we did not include this vaccine in our meta-analysis due to the insufficient data provided in current publications.30 We expect more details to be provided in the future.
Second, tumour vaccines presented enhanced tumour antigens. Lung tumour cells expressed non-classical HLA (human leucocyte antigen) class I molecule, HLA-G, which has direct inhibitory effects on T-cell, antigen-presenting cell (APC) and NK function, with induced suppressor cells. Besides, the low expression of major histocompatibility complex I (MHC-I) molecules on lung tumour cells lead to weak antigen presentation.31 Current tumour vaccines are coupled with immunogenic adjuvant agents with tumour-specific antigens. The potential strong adjuvant agents enhance the APC response, which activates tumour-specific T cells to strengthen the immune response.32 One strategy is genetically modifying autologous tumour cells or allogeneic cell lines, or genes modified to secrete cytokines and/or co-stimulatory molecules and antigens expressed in a viral vector.27 In our analysis, the therapeutic outcomes of GVAX vaccine were assessed.20 Another strategy is non-antigen-specific immunomodulatory agent-promoted co-stimulatory molecules.33 SRL172 and TLR 9 vaccines activate antigen-presenting cells and enhance maturation of plasmacytoid dendritic cells (pDCs), which increase cell-surface expression of MHC-II molecules, as well as activate the co-stimulatory molecule B7 and CD8+ cytotoxic lymphocyte (CTL).34
Third, tumour vaccines prevented immune tolerance. Lung tumour cells produced a variety of immunosuppressive molecules to induce immune tolerance, including transforming growth factor β (TGF-β), prostaglandin E2, IL-10 and cyclooxygenase 2. TGF-β2 blocked maturation of DCs that affect DC processing and presentation, as well as promoted T regulatory cells to suppress immune responses.35 Belagenpumatucel-L vaccine is an allogeneic irradiated NSCLC cell line transfected with TGF-β antisense transgene, which inhibits the expression of TGF-β.36 However, the phase II Belagenpumatucel-L trial is not RCT, and the phase III clinical results is published on EMSO 2013 as an abstract, and more details are needed.37
Fourth, all the clinical therapeutic outcomes in our selected papers were assessed by chemotherapy RECIST criteria, which evaluated the effect of cytotoxic agents.25 In contrast, immune-related response may take much longer to become clinically obvious, and may even sometimes lead to temporary enlargement in a lesion before it shrinks.38 However, most of the published clinical data were short of specific immune-related response criterion. Therefore, our analysis may introduce bias assessment of the clinical activities of tumour vaccine immunotherapy, such as immunological assessment of T-cell proliferation and tumour marker expression.
Tumour vaccines have a relatively low toxicity profile compared with other oncological therapies. Some side effects such as nausea and vomiting, thrombocytopaenia and injection site reaction (p≤0.05), had a significant impact on the tumour vaccine-treated group in our study. In our analysis, patients receiving vaccine treatment experienced a few severe side effects (grades ≥3), but no statistical difference was observed compared with the corresponding control group (figure 5). Other research also indicated tumour vaccine therapy resulted in fewer AEs and presented less toxicity compared with other immunotherapies, including mAbs.39 The evaluation of tumour vaccine safety is not only dependent on the vaccine itself, but also influenced by treatment regimen, dosage, administration protocols and patients’ selective criteria. Thus, tumour vaccine with chemotherapy has proven to be a feasible and effective immunotherapy for the treatment of NSCLC without severe side effects.
Taken together, NSCLC tumour vaccines markedly prolong survival rates (median OS, p<0.00001), TTP (p=0.001) and PFS (p=0.005), improve clinical response rate (p=0.05) and lessen adverse side effects (p<0.00001). Our meta-analysis suggests tumour vaccines improve the efficacy of treatment, and also provide superior treatment of patients with advanced NSCLC among a variety of immunotherapy strategies. Immunotherapeutic approaches in the treatment of NSCLC were combined with standard chemotherapy or radiotherapy. Based on the mechanism of tumour vaccine, chemoradiotherapy may generate synergistic effects. However, to optimise the efficacy and safety of tumour vaccines, immunotherapy timing, regimen, dosage and administration protocols are still required for further research. We look forward to larger scale clinical RCT results in future publications. Thus, we hope our analysis will provide valuable evidence for the evaluation of tumour vaccine therapy. Tumour vaccine therapy may become a standard complementary therapeutic treatment for advanced NSCLC.
Acknowledgments
The authors appreciated Jian-hong Pan for providing advices on statistical analysis (Department of Biostatistics, Peking University Clinical Research Institute, Peking University Health Science Center, Beijing, China).
References
Footnotes
Contributors Z-X W designed the research; MW and J-XC performed the research; Y-SL and DL contributed analytical tools; MW, B-LX, X-YZ, J-LL, J-LL and H-BW analysed the data; MW and J-XC wrote the paper.
Funding This research work was supported by the Postdoctoral Foundation of China (No. 2014M552694 to Min Wang).
Competing interests None declared.
Patient consent Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement No additional data are available.